Journal of
eISSN: 2469 - 2786


Research Article Volume 3 Issue 4
1Industrial Research and Consultancy Centre, Sudan
2University of Khartoum, Sudan
Correspondence: El Rasheed Ahmed Salim, Industrial Research and Consultancy Centre, Ministry of Industry, Sudan, Tel 00249912204672
Received: September 21, 2016 | Published: December 22, 2016
Citation: Salim ERA, Yagi S, Elyass HMM. Histology, phytochemistry and bacterial activity of anise (Pimpinella anisum L.) seed and essential oil. J Bacteriol Mycol Open Access. 2016;3(4):278-282 DOI: 10.15406/jbmoa.2016.03.00070
This study was conducted to investigate the histology, phytochemistry and antimicrobial activities of anise (Pimpinella anisum L.) seed and essential oil obtained from Omdurman market, Sudan. Histology was conducted by preparations, fixation, dehydration, clearing, wax embedding, sectioning, staining and mounting. Moisture content performed according to FAO manuals. Oil extraction according to British pharmacopeia. Test of the oil for antimicrobial activity conducted by cup-plate agar diffusion method adopted, with some minor modification to assess the antibacterial activity of the prepared extracts. Standard microorganisms namely; Staphylococcus aureus ATCC 25923 Gram (+ve) bacteria, Klebsiella pneumoniae ATCC 53657 Gram (-ve) bacteria, Escherichia coli ATCC 25922 Gram (-ve) bacteria and Pseudomonas aeruginosa ATCC 27853 Gram (-ve) bacteria were obtained from Medicinal and Aromatic Plant Institute, National Centre of Research, Sudan. Two concentrations used A (0.5ml of anise essential oil in 4.5ml solvent (methanol) to form (1:9) ratio and Concentration B (2.5ml of concentration diluted with 2.5ml solvent (methanol) to form (1:18) ratio). Histological features of anise under microscopical examining showing hair (hr), epidermis (epi), mesocarp (mes), oil vittae (vit), endocarp (endc), cuticle (cu), endosperm (ed), fibrovascular bundle (fb), sclerenchyma (sch), srystals (cry) and fixed oil (fo). Phytochemical investigation of anise showed purity (69.5%), moisture content (6.7%), oil content (1.5% (v/w)), refractive index (1.5622), specific gravity (0.9825), odor and taste (anisthole odor), acid value (1.29) and ester value (14.03). Microbiological activities of anise oil using concentration A and B against Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa showed moderate antibacterial effect. Concentration A showed low inhibition and Concentration B showed no antimicrobial activities against Escherichia coli.
Keywords: anise essential oil, histology, staphylococcus aureus, klebsiella pneumonia, escherichia coli, pseudomonas aeruginosa,physico-chemicals
Anise (Pimpinella anisum L.) (family: Umbelliferae) origin is mediterranean region.1 The major production area in Sudan is Northern Sudan, while there is a very limit production in Khartoum state.2 Aniseeds contain 1.5-5% essential oil and used as flavouring, digestive, carminative, and relief of gastrointestinal spasms. Consumption of aniseed in lactating women increases milk and also reliefs their infants from gastrointestinal problems.3 In the food industry, anise is used as flavoring and aromatic agent for fish products, ice cream, sweets, and gums.4,5 The composition of anise varies considerably with origin and cultivation method. These are typical values for the main constituents; Moisture: 9-13%, Protein: 18%, Fatty oil: 8-23%, Essential oil: 2-7%. Starch: 5%, N-free extract: 22-28%, Crude fibre: 12-25%.6 Essential oil yielded by distillation is generally around 2-3% and anethole makes up 80-90% of the oil.6 Anise contains anethole, a phytoestrogen.7 The essential oil has reportedly been used as an insecticide against head lice and mites. The oil is very famous oil throughout the world. It was used in confectioneries, pharmaceutical, tooth paste, and other industrial uses.8
Anatomical features of anise are a vital process for clear identification of the plant, and further investigations in plant anatomy, phytochemistry and other recommendations. Phytochemical studies of anise oil is important for identification, quality control and quality assurance and other uses of essential oil for medicinal and food purposes. Due to variation in climate, topography, soil, and cultural practices; anise essential oil can be differ, therefore more investigation are important to clarify this changes. Morphological, structural and developmental features of fruits and seeds are here studied, with the purpose to give a proper classification of their fruit and embryo type and to contribute to future taxonomical and ecological studies.
Furthermore, some spices are reported to have bactericidal or bacteriostatic activities. The inhibitory effects of spices are mostly due to the volatile oils present in their composition.9 The main factors that determine the antimicrobial activity are the type and composition of the spice, amount used, type of microorganism, composition of the food, pH value, temperature of the environment, and proteins, lipids, salts, and phenolic substances present in the food environment.10
The objectives of this study are to investigate histology, phytochemistry and antibacterial activities of anise oil on Staphylococcus aureus, Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa using two concentrations (1:9 and 1:18).
Source of the plant
Anise seed was obtained from local Omdurman market, Sudan. The production is area is the northern region of Sudan. The original plant source was suggested to be brought from Egypt far a year ago through River Nile valley movement.
Histology
Preparations: Soft tissue method adopted; seeds soaked into running water for two days and then run in calm water not exceed a temperature of 60˚C.
Fixation: Seeds were collected into formaline: acetic acid: alcohol (FAA) (5:5:90v/v). The fixative was changed repeatedly till the solution looked transparent, and calmed in an oven at 60˚C. The heating liquefies oil contents in glands, so that to insure complete leaching of the oil from the plant.
Dehydration: Seeds were dehydrated through passing them into a series of concentrations of ethyl alcohol from 50 to 95%.
Clearing: The seeds and stems after dehydration cleared by passing them in a mixture of solutes; ethyl alcohol: cedar oil (50:50%), cedar oil: xylene (50:50v/v) for at least 6 hours (incubation) and the thirdly through pure xylene for overnight respectively in 60˚C.
Wax embedding: The above specimens were transferred from xylene and parafine to pure wax to form blocks.
Sectioning: The waxed specimens were sectioned using a rotatory microtome (Letize 1512 - West Germany).
Staining: Dewaxing of sectioned specimens by xylene several times to insure dewaxing. Then hydration of the dewaxed specimens using decreasing dilutions of alcohol from 95 to 50%, followed by immersing in hematoxylene for 60 minutes, then finally washed by running water for 15 minutes. Then again dehydration was conducted by passing the specimens through ammonified water (4%) for 30 seconds, followed by passing through increasing concentrations of ethyl alcohol and finally transfers to xylene.
Mounting: The prepared sections were mounted on Canada balsam, and then the sections were covered and placed immediately on hot horizontal surface in the oven at 60˚C for 72 hours before microscopical examining.
Determination of moisture content
Moisture content was determined according to FAO manuals.11
Determination of oil Content (v/w)
Anise crushed to powders using a grinder. Essential oil was obtained by hydrodistillation of the powdered dry fruits according to the British Pharmacopeia Protocol. The oil phase was separated, dried over anhydrous sodium sulfate, and kept in a dark glass bottle at 4˚C until the analyses.12
Physical and chemical properties
Physical and chemicals properties were determined according to BS207313 except odour and volatilization according to British pharmacopeia.12
Preparation of extracts
Dilution of 0.5 ml of anise essential oil in 4.5 ml solvent (methanol) (1:9) to make Concentration A., 2.5 ml of concentration A was further diluted with 2.5 ml solvent (methanol) (1:18) to make concentration B.
Source of microorganisms
Four standard organisms namely; Staphylococcus aureus ATCC 25923 Gram (+ve) bacteria, Klebsiella pneumoniae ATCC 53657 Gram (-ve) bacteria, Escherichia coli ATCC 25922 Gram (-ve) bacteria and Pseudomonas aeruginosa ATCC 27853 Gram (-ve) bacteria were obtained from Medicinal and Aromatic Plant Institute, National Centre of Research, Sudan.
Test of extracts for antimicrobial activity
To determine the effectiveness of anise oil against the above four organisms’ cup-plate agar diffusion method was adopted, with some minor modification to assess the antibacterial activity of the prepared extracts.14 Two ml of the standard bacterial stock suspension (10-10) colony forming units per ml were thoroughly mixed with 200 ml of sterile nutrient agar which was maintained at 45˚C, 20 ml aliquots of the inoculated nutrient agar distributed into sterile petridishes. Alternate cups were filled with 0.1 sample of each of the concentrations using standard Pasteur pipette and allowed to diffuse at room temperature for two hours. The diameter of zone of inhibition (mean of two replicates ± SD) as indicated by clear area which was devoid of growth of microbes was measured to determine the antibacterial activity. Two replicates were carried out for each extract against each of the test organisms. After incubation the diameter of the resultant growth inhibition zones were measured, averaged and the mean value was tabulated. The experiment was replicated two times to confirm the reproducible results.
Histology
Anise histology with respect to oil structure, endodermis, and fibrovascular bundle were shown in Figure 1. Oil vittae were located embedded between the external epidermis of the seed and mescarp. Other anatomical structures such as endodermis and vascular bundle were occurred. Figure 2 should be anise seed; the closer view showing the structure of oil bearing structure in spices; the oil vittae. This finding agreed with Abu-zeid1 who said that oil secretory structure of anise seeds was found in secretory structure known as vitta. Also the above results were coinciding with Parry15 who illustrated the presence of oil vittae in the mesodermis of anise seeds Plates 1-4. The fruit "rind" consists of the exocarp, represented by a periderm with lenticels, and by the parenchymatic mesocarp, with branched secretory ducts and vascular bundles.16 The edible pulpy is formed by the endocarp, destituted of secretory ducts, and derived from the activity of a ventral meristem, which emerges early in the fruit development.16 The inner endocarp cell layers undergo a radial elongation and become firmly attached to the testal outer layers.16 Figure 3 showed a cross section on anise seed to give more details about the oil vitae. Oil vittae was located embedded between the external epidermis of the seed and mesocarp other anatomical structures such as vascular bundle was occurred. Oil vittae embedded between the external epidermis of the seed and mesocarp. Other anatomical structures were such as endocarp, sclerenchyma, cuticle, endosperm, sclerenchyma, crystals and fixed oil. The presence of oil secretory structure in the mesoderm of anise seeds agreed with Parry15 who illustrated the presence of oil glands on stem exodermis of mint leave.
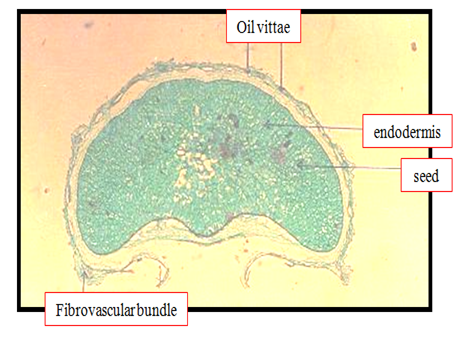
Figure 1 Transverse section (10X) through the anise seed showing endodermis, oil vittae (vit) and fibrovascular bundle (fb).
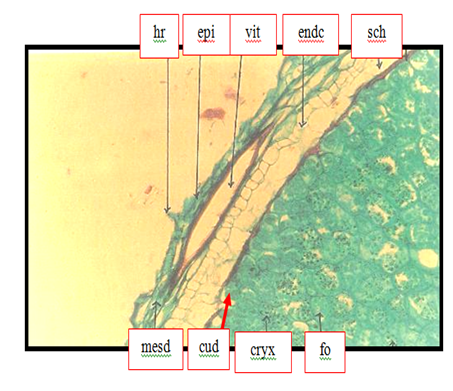
Figure 2 Transverse section (40X) through the anise seed showing hair (hr), epidermis (epi), mesocarp (mes), oil vittae (vit), endocarp (endc), cuticle (cu), endosperm (ends), sclerenchyma (sch), crystals (cryx) and fixed oil (fo).
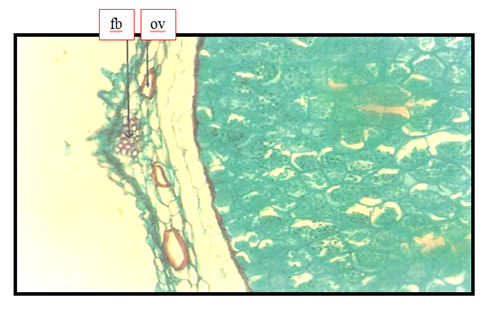
Figure 3 Transverse section (40 X) through the anise seed showing oil vittae (vit) and fibrovascular bundle (fb).
Phytochemistry
Purity, moisture content, oil content, refractive index, specific gravity, odor and taste, acid and ester values of fresh herb of anise were shown in Table 1. Moisture content was less than what mentioned by Pruthi.6 Oil content of anise was 1.5 % which is at a minimum of the range determined by Guenther8 who reported that anise oil ranged between 1.5 - 6.0%. The reduction may be due to climatic, cultural or post harvest conditions. Refractive index 1.5622 which higher than what mentioned by Guenther8 which ranged between 1.5520 to 1.5590. Specific gravity of anise oil was 0.9825 and fell in the range mentioned by Guethner8 which ranged between 0.980 - 0.990. The oil odour was similar to anithole; which constitute the major party of anise oil; indicating that this oil is truely anise oil. Evaporation test stated that no fixed oil was present in anise oil (Figure 4).
Properties |
Values |
Purity |
69.50% |
Moisture content |
6.70% |
Oil content |
1.5% (v/w) |
Refractive index |
1.5622 |
Specific gravity |
0.9825 |
Odor and taste |
Anithole odor |
Evaporation |
No trace left |
Acid value |
1.29 |
Ester value |
14.03 |
Table 1 Purity, moisture content, oil content, physical and chemical properities of anise seed and oil

Figure 4 Cross section through anise seed showing oil vittae and other histological structure.15
Acid value and ester value of anise oil were 1.29 and 14.03 respectively and they are lower than what mentioned by Guenther8 who mentioned that anise acid value and ester value were 5.83 and 16.83 respectively, this may be attributed to climate, cultural practices and post harvest factors. It is well documented that genetic constituents and environmental factors influence the yield of volatile oils produced by medicinal plants.17-19
Microbiology
Antibacterial effects of anise oil both concentrations (1:9) and (1:18) was illustrated in Table 2. The results showed that concentration A (1: 9) of anise seed oil showed moderate inhibitory effect against Staphylococcus aureus ATCC 25923 Gram (+ve) bacteria. And concentrations B of anise seed oil (1:18) had low effect against Staphylococcus aureus ATCC 25923 Gram (+ve) bacteria.
Microorganisms |
Inhibition zones (mm) mean |
|
Concentration A |
Concentration B |
|
Staphylococcus aureus |
13.5 (M) |
15 (L) |
Klebsiella pneumoniae |
17 (M) |
17.5 (M) |
Echerichia coli |
14 (L) |
- |
Pseudomonas aeruginosa |
15.5 (M) |
15.5 (M) |
Table 2 Antibacterial activity of anise volatile oil using two diluted concentrations
Where:
Test volume of extract = 0.1 ml/cup.
18 mm > high (H). 15-17mm moderate (M), 12 - 14mm low (L) and 11< none (N).
Concentration A and B showed moderate inhibitory effect against Klebsiella pneumoniaeATCC 53657 Gram (-ve) bacteriaand Pseudomonas aeruginosa ATCC 27853 Gram (-ve) bacteria. While Concentration A (1:9) of anise seed oil showed low inhibitory effect against Escherichia coliATCC 25922 Gram (-ve) bacteria.These results partially agree with Gangrad20 who mentioned that anise essential oil has antibacterial activity against Staphylococcus aureus and Escherichia coli.
We can conclude that the anatomy, phytochemistry and microbiology of anise seed and oil postulates the followings; Anatomy of aniseed exhibits the nominal histological structures of anise seed likes the oil vittae, hair, epidermis, mesocarp, endocarp, cuticle, endosperm, sclerenchyma, crystals and fixed oil which provide histological structures for identification of Sudanese aniseseed. The physicochemical properties of the oil gave good quality of anise oil and reflected identification of their pharmacognosy properties. Antibacterial activity of anise essential oil was reported against Staphylococcus aureus ATCC 25923 Gram (+ve) bacteria, Klebsiella pneumoniae ATCC 53657 Gram (-ve) bacteria, Escherichia coli ATCC 25922 Gram (-ve) bacteria and Pseudomonas aeruginosa ATCC 27853 Gram (-ve) bacteria.
I wish to express my thanks to the staff of Medicinal and Aromatic Plants Institute (NCR, Sudan) for their support during tests antimicrobial. My thanks extend to Department of Botany, Faculty of Science, and University of Khartoum for their assistance in anatomical work to complete this study with special thanks to Mr. Kamel. Also my thanks due to Department of Food Research Industries, Industrial Research and Consultancy, Sudan for their generous help in conducting extraction and chemical tests.
The author declares no conflict of interest.

©2016 Salim, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.