Journal of
eISSN: 2473-0831


Research Article Volume 5 Issue 2
Correspondence: Pravin Shende, Shobhaben Pratapbhai Patel School of Pharmacy and Technology Management, SVKM?s NMIMS, Vile Parle (W), Mumbai (MS) 400056, India
Received: April 26, 2017 | Published: June 26, 2017
Citation: Atrey E, Shende P, Gaud RS (2017) RP-HPLC Method Development and Validation for Simultaneous Estimation of Ondansetron Hydrochloride and Complexed Famotidine in Bulk and Dosage Form. J Anal Pharm Res 5(2): 00138. DOI: 10.15406/japlr.2017.05.00138
A simple, economical, selective, rapid and precise HPLC method has been developed for simultaneous estimation of ondansetron hydrochloride and famotidine in their bulk and pharmaceutical dosage forms. The study was carried out using a column of Kromasil 100-C8 (250mm×4.6mm, 5µm) with a mobile phase consisting degassed mixture of phosphate buffer and methanol (50:50) at a flow rate of 0.6ml/min. The retention time of ondansetron hydrochloride and famotidine at wavelength 250 nm was found to be 7.74 and 4.44min, respectively. This developed method was also validated for linearity, accuracy, system suitability and robustness.
Keywords:ondansetron hydrochloride, complexed famotidine, HPLC, soluble film
Ondansetron is 9-Methyl-3-.(2-methyl-1H-imidazol-1-yl) methyl-1, 2, 3, 9-tetrahydro-4H-carbazol-4-one acted by inhibition of 5-HT3 receptor presently both centrally and peripherally (Figure 1). It is used to prevent and treat chemotherapy-induced nausea and vomiting as it has both anxiolytic and neuroleptic properties.
Famotidine is histamine H2-receptor antagonist which is chemically 3-.[[2-[(aminoiminomethyl) amino-4thiazolyl] methyl] thio]-N-(aminosulfonyl) propanimidamide acted by inhibiting gastric acid production and secretion by reversibly competing with histamine for binding to H2-receptors on the basolateral membrane of parietal cells, decreases both basal and food stimulated acid secretion by 90% or more, but also promotes healing of duodenal ulcers (Figure 2).
Complexation has gained good acceptance in recent years for enhancing the solubility, dissolution rate and bioavailability of the drug as it gets completely absorbed in gastric medium. Hence, complexation technique plays a sophisticated role to find its way in area of expectations during the process of formulation development.1
The development of fixed dose combination has become increasingly important from public health perspective view and the use of this fixed dose combinational formulation helps to lower the cost of drug when compared with the cost of individual drug formulation. Combination of ondansetron hydrochloride and famotidine has the advantages of greater therapeutic effects than with either drug alone. This combination causes manifold reduction in emesis and acid secretion in gastric level as compared to double dose of the individual drug when used alone.
The pharmaceutical importance of famotidine and ondansetron hydrochloride combinational work concerns with development and validation of two simple, sensitive and selective HPLC methods for determination of the proposed drugs in their pure forms and combined dosage form. Gastroesophageal reflux disease (GERD) is a common chronic disorder prevalent in many countries. Apart from the economic burden of the disease and its associated impact on quality of life, it is the most common predisposing factor for adenocarcinoma of the esophagus.2-4 GERD is associated with regurgitation leading to sour taste in the mouth. The taste, along with the frequent burping and coughing associated with GERD, leads to nausea and even vomiting. Indigestion is another symptom of GERD that can contribute to nausea .5 Acid suppression is the main line of treatment for GERD. As it is often associated with nausea and vomiting, a good antiemetic would be useful in the management of patients with GERD. To manage these symptoms of GERD, we have made a fixed dose combination of famotidine and ondansetron mouth dissolving strips. Famotidine will suppress the excess acid secretion which is the cornerstone in the pathophysiology of GERD and ondansetron will be beneficial in relieving the symptoms of nausea and vomiting in these cases.6,7 As the patients with GERD already have nausea and vomiting, an oral tablet shall further increase these symptoms thus we have made a mouth dissolving strip which would be readily absorbed by the buccal mucosa directly in the blood stream and would thus bypass the GI tract, preventing the potential trigger for another episode of nausea and vomiting and also ensuring rapid onset of action of the drugs. Moreover, this mouth dissolving strip would also be a convenient mode of drug delivery for patients of geriatric and pediatric age group who have problem in swallowing tablets. Thus this novel combination of complexed famotidine and ondansetron mouth dissolving film would be useful in the management of GERD patients. The objective of the study was to develop RP-HPLC method and validate for simultaneous estimation of ondansetron hydrochloride and complexed famotidine in bulk and dosage form.
Instrumentation
The HPLC system (Agilent, USA) equipped with a 1200 binary pump and a photodiode array detector was used for the study. The separation was carried out using a Kromasil C8 (250mm×4.6mm, 5µm) and a mobile phase comprising of potassium dihydrogen phosphate with a pH of 6.8 and methanol in a ratio of 50:50. The mobile phase was filtered and degassed by sonication and was pumped at a flow rate of 0.6mL/ min, injection volume of 20µl , run time of 12 min and the column temperature was maintained at 40°C throughout the analysis. The chromatograms were detected at wavelength of 250nm.
Chemicals and reagents
Ondansetron hydrochloride was obtained from IPCA laboratories, Mumbai, India and Famotidine was obtained from Mahashree Laboratories Private Limited, Ankleshwar, India. All other chemicals and reagents used in the study were of analytical grade.
Preparation of mobile phase
A filtered and a degassed mixture of phosphate buffer pH 6.8 and methanol was prepared in a ratio of 50:50 v/v for the study.
Preparation of standard stock solution
Ondansetron hydrochloride and famotidine were weighed 40mg and 50mg respectively and transferred to a 100ml volumetric flask. 50ml of diluent was added and sonicated to dissolve. Volume was made up to the mark with the diluent and mixed. The stock solution was diluted to obtain test concentration of 40µg/ml and 50µg/ml of ondansetron hydrochloride and famotidine respectively.
Preparation of oral soluble film sample
Four cm2 of the film containing 10mg of famotidine and 8 mg of ondansetron hydrochloride was transferred into a screw capped 100ml glass bottle containing 100ml of diluent added quantitatively and vortexed for about 2min on vortex cycling mixer. The content was sonicated for 1h, vortexed for 2min on vortex cycling mixer and allowed to cool at ambient temperature. Ten ml of the solution was diluted to 20.0ml with the diluent and mixed. The solution was filtered through 0.45µm Millipore PVDF filter and the filtrate was collected by discarding first few ml of the filtrate and the resulting sample solution was subjected to HPLC analyses.
Development and optimization of HPLC method.8,9
The HPLC method was optimized with an aim to develop a simultaneous estimation procedure for the famotidine and ondansetron hydrochloride.
Method validation of RP-HPLC method10–13
The developed method was validated according to ICH guidelines for linearity, accuracy, LOD and LOQ, precision, system suitability and robustness. To check the system performance, the system suitability parameters were measured. System precision was determined on five replicate injections of standard preparations. Number of theoretical plates was measured.
Linearity: Accurately weighed quantity of famotidine and ondansetron hydrochloride was transferred into a 200ml volumetric flask, dissolved and volume was made up with diluents and mixed. Calibration curves were constructed by plotting peak area against concentration of ondansetron hydrochloride and famotidine and the regression equations were calculated. The calibration curves were plotted over 9 different linear concentrations in the range of 5-150µg/ml for famotidine and 4-120µg/ml for ondansetron hydrochloride.
Accuracy: The accuracy of the test method was demonstrated by preparing recovery samples in screw bottle at the three levels of 80%-120% concentrations of each drug in formulation. The recovery samples were prepared in triplicate in each level. The film was transferred into a screw bottle containing specified amount of stock solution and diluents, sonicated for 1 h and 10ml of the solution was diluted to 20ml with the diluents and filtered through 0.45µm Millipore PVDF filter. The filtrate was then collected by discarding first few ml of the filtrate. The above samples were chromatographer and percentage recovery for the amount added was calculated.
System suitability: System suitability is an integral part of method development that verifies whether the system is adequate for the analysis of famotidine and ondansetron hydrochloride. System suitability of chromatography system was performed before each validation run. Five replicate injections of system suitability were prepared and % RSD were determined.
System precision: The precision of the HPLC system was established for the analysis. The repeatability studies were carried out by estimating the response of ondansetron hydrochloride and famotidine five times and results were reported in terms of relative standard deviation.
Limit of detection (LOD) and limit of quantitation (LOQ): The LOD and LOQ of the drug were calculated by using the following equations:
Where, ‘σ’ is the standard deviation of the response and ‘S’ is the slope of the calibration curve
Robustness: The purpose to establish the robustness method was to demonstrate its reliability for minor changes in chromatographic conditions and carrying out system suitability under normal condition and each of altered conditions such as:
Optimization of mobile phase
The method development was used to resolve the chromatographic peaks for both active drug ingredients with less asymmetric factor and the mobile phase phosphate buffer (pH 6.8): methanol in a ratio of 50:50. It was found to be satisfactory that showed two symmetric and well resolved peaks of ondansetron hydrochloride and famotidine. The retention time of ondansetron hydrochloride and famotidine was found to be 7.74 min and 4.44min, respectively with a resolution of 16.62 which indicates good separation of both the compounds detected at 250nm as shown in Figure 3.
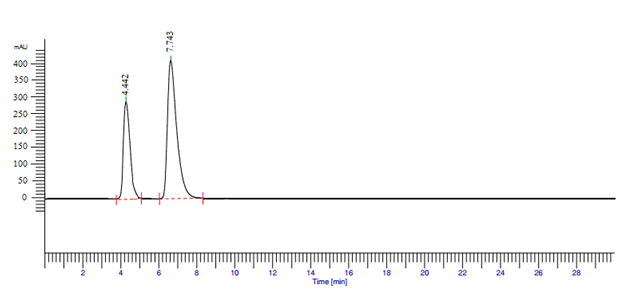
Figure 3 Simultaneous estimation chromatogram of ondansetron hydrochloride and famotidine in combinational dosage form.
Method validation
Linearity and calibration curves: The calibration curve of ondansetron hydrochloride was found to be linear in range of 4-120µg/ml with correlation coefficient of 0.999 whereas the calibration curve of famotidine was found to be linear in range of 5-150µg/ml with correlation coefficient of 0.999 as shown in Figures 4 &5.
Accuracy: The % recovery of famotidine and ondansetron hydrochloride was within acceptance criteria given in ICH guideline as shown in Table 1.
|
Sr. No. |
Amount Added (mg) |
Amount Found (mg) |
% Recovery |
% RSD |
||||
|
Famotidine |
Ondansetron Hydrochloride |
Famotidine |
Ondansetron Hydrochloride |
Famotidine |
Ondansetron Hydrochloride |
Famotidine |
Ondansetron Hydrochloride |
|
|
1 |
10.02 |
8.012 |
9.8588 |
7.8806 |
98.4 |
98.4 |
1.5 |
1.6 |
|
2 |
10.02 |
8.012 |
10.1592 |
8.1385 |
101.4 |
101.6 |
||
|
3 |
10.02 |
8.012 |
10.0211 |
8.0279 |
100 |
100.2 |
||
|
Mean |
10.013 |
8.015 |
100.1 |
99.9 |
||||
Table 1 Accuracy data for famotidine and ondansetron hydrochloride
System suitability: The column efficiency was not less than 2000 theoretical plates, resolution was 16.2 and the tailing factor for analyte peak was 0.216 for famotidine and 0.353 for ondansetron hydrochloride drugs. The study concludes the suitability of the HPLC system as shown in Table 2.
|
Analyte |
Retention Time (min) |
Tailing Factor (T) |
Theoretical Plates (N) |
Resolution® |
|
Famotidine |
4.47 |
0.216 |
11691 |
- |
|
Ondansetron Hydrochloride |
7.994 |
0.353 |
16248 |
16.2 |
|
Required Limit |
- |
T<2 |
N>2000 |
R>2 |
Table 2 System suitability data for famotidine and ondansetron hydrochloride
System precision: As shown in Table 3 & 4 the % RSD for repeatability of both the drugs was found to be less than 2. So, it was observed that proposed method for estimation of ondansetron hydrochloride and famotidine is précised in nature.
|
Parameter |
Famotidine |
Ondansetron hydrochloride |
|
SD |
1.64 |
3.5 |
|
%RSD |
0.08 |
0.1 |
Table 3 Reading of system precision
|
Parameter |
Famotidine |
Ondansetron hydrochloride |
|
Mean Slope |
37.3805 |
81.018 |
|
LOD |
0.14478 |
0.1425 |
|
LOQ |
0.438 |
0.432 |
Table 4 Reading of for LOD and LOQ
Robustness: The robustness of the method was estimated by assaying test solutions under different analytical conditions deliberately changed from the original conditions like mobile phase composition, flow rate, temperature conditions, etc. and the mean and % RSD was reported for famotidine and ondansetron hydrochloride in Tables 5 & 6, respectively.
|
Parameters |
Temperature conditions |
Flow rate |
Mobile phase changes |
|||
|
35°C |
45°C |
10% |
-10% |
2% |
-2% |
|
|
Mean |
3241.32 |
3251.59 |
2781.67 |
3892.41 |
3252.61 |
3255.72 |
|
%RSD |
0.12 |
0.05 |
0.1 |
0.16 |
0.2 |
0.13 |
Table 5 Robustness for ondansetron hydrochloride
|
Parameters |
Temperature conditions |
Flow rate |
Mobile phase changes |
|||
|
35°C |
45°C |
10% |
-10% |
2% |
-2% |
|
|
Mean |
1874.87 |
1872.24 |
1604.87 |
2247.46 |
1861.08 |
1891.38 |
|
% RSD |
0.1 |
0.11 |
0.13 |
0.13 |
0.17 |
0.2 |
Table 6 Robustness for famotidine
Analysis of marketed formulation: The drug content of famotidine and ondansetron hydrochloride in an oral soluble film was found to be 96.2% and 98.89% respectively. The % content of drugs in the marketed formulation was found to be as follows
Famotidine
Area of Standard= 1947.41003.
Area of Test= 1873.57556.
Ondansetron hydrochloride
Area of Standard= 3285.60645
Area of Test=3249.42749
=96.2%
=98.89%
From the results, a simple and validated stability indicating HPLC method was developed for simultaneous estimation of ondansetron hydrochloride and famotidine. It was observed that the developed method is specific, accurate, precise and robust. The method showed a linear response in stated range and is accurate and precise. Moreover, low limit of quantitation and limit of detection made this method suitable for the use in quality control.
None.
The authors declare no conflicts of interest related to this article.
None.

©2017 Atrey, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.