Journal of
eISSN: 2473-0831


Research Article Volume 7 Issue 2
Correspondence: Hassouna MEM, Department of Chemistry, Faculty of Science, Beni-Suef University, Beni-Suef 62514, Egypt, Tel +2 01223861504
Received: February 18, 2018 | Published: March 7, 2018
Citation: Hassouna MEM, Mohamed MA, Novel and facile spectrophotometric techniques for the determination of sofosbuvir and ledipasvir in their tablet dosage form. J Anal Pharm Res. 2018;7(2):90 – 97. DOI: 10.15406/japlr.2018.07.00207
The aim of this work is to develop and validate three accurate, simple, selective and specific spectrophotometric methods for the determination of Sofosbuvir (SOF) and Ledipasvir (LDV) in pure and in their dosage forms. LDV can be easily determined in the zero order at a wavelength of 333 nm with good correlation coefficient (0.9997), with no interference from SOF. While the latter could be determined in presence of LED by three novel spectrophotometric methods viz., (I) first derivative (1D), (II) ratio difference (RD) and (III) ratio subtraction (RS). In method (I) SOF has λmax at 274nm with good correlation coefficient (0.9997). In method (II), laboratory prepared mixtures are divided by the absorption spectrum of standard 10μg/mL LDV for the determination of SOF and the ratio spectra are recorded at 208nm and 270nm for SOF with good correlation coefficient (0.9993). Finally, in the third one (III), ratio subtraction method is established by dividing the spectrum of the binary mixture by the standard spectrum of 25μg/mL LDV as a divisor and subtract the constant value determined in the plateau region at 290–375nm, then multiply by the divisor to obtain zero order (D0) original spectrum of SOF at 261nm with good correlation coefficient (0.9994). Accuracy, recovery and the selectivity of the developed methods are confirmed by applying the standard addition technique, testing on authentic mixtures and application on pharmaceutical dosage forms. The obtained results are statistically compared with those obtained from the recently published HPLC method for their simultaneous determination using one-way analysis of variance (ANOVA) where no significant difference was observed between the proposed methods and the well-established ones which prove their validity for the analysis of this binary mixture.
Keywords: sofosbuvir, ledipasvir, first derivative method, ratio subtraction method (RS), ratio difference method (RD)
HCV, hepatitis C virus; EDHS, Egyptian Demographic Health Survey; HIV: Human Immuno Deficiency Virus; FDC: Fixed-Dose Combination; IS: Internal Standard; AD: Alcohol Dependence; RSM: Ratio Subtracting Method; RD: Ratio Difference; RS: Ratio Subtraction
Between 130–150 million people in the Glob have chronic hepatitis C virus (HCV) infection. A significant number of those who are chronically infected will develop liver cirrhosis or liver cancer. Approximately 700 000 people die each year from hepatitis C-related liver diseases.1 Unfortunately, Egypt has the largest epidemic of HCV in the world according to the released Egyptian Demographic Health Survey (EDHS) and the overall prevalence (percentage of people) positive for antibody to HCV in Egypt was 14.7%.2–4 The current population in Egypt is about 100 millions, thus ≈14.7 millions of persons who have been infected with this virus. Gilead Sciences overcome most common related liver diseases by its Great invention (Harvoni). Harvoni (90mg ledipasvir/400mg Sofosbuvir) initially approved by United States FDA in 2014 and expanded indications are approved by FDA in 2015. It is indicated for the treatment of chronic HCV genotypes 1, 4, 5, and 6 in adults and also indicated for the treatment of chronic HCV in patients co-infected with Human Immuno deficiency Virus (HIV).4
SOF is chemically known as (S)-Isopropyl 2-((S)-(2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl) methoxy)-(phenoxy)phosphoryl amino) propanoate. It has a molecular formula of C22H29FN3O9P and a molecular weight of 529.45 (Figure 1). It is a white to off-white powder with a solubility of ≥2mg/mL across the pH range of 2-7.7 at 37°C. The partition coefficient (log P) for Sofosbuvir is 1.62 and the pKa is 9.3.5
Sofosbuvir is a pangenotypic inhibitor of the HCV NS5B RNA-dependent RNA polymerase, which is essential for viral replication.6 It is a nucleotide prodrug that undergoes intracellular activation to form GS-461203 (active triphosphate, not detected in plasma), and ultimately the inactive, renally eliminated metabolite GS-331007.7 The pharmacologically active uridine analog triphosphate (GS-461203) can be incorporated by HCV NS5B and acts as a chain terminator. In a biochemical assay, GS-461203 inhibits the polymerase activity of the recombinant NS5B from HCV genotype 1b, 2a, 3a and 4a with an IC50 value ranging from 0.7 to 2.6µM. GS-461203 is neither an inhibitor of human DNA and RNA polymerases nor an inhibitor of mitochondrial RNA polymerase.8 In 2013, the FDA approved SOF, in combination with RBV, with or without PEG-IFN depending on genotype, is recommended for HCV GT 1, GT 2, GT 3 and GT 4.9,10 Sofosbuvir/Ledipasvir is a fixed-dose combination (FDC) tablet containing sofosbuvir (previously approved NS5B polymerase inhibitor) and ledipasvir, a new NS5A-inhibitor.5
LDV is chemically known as Methyl [(2S)-1-{(6S)-6- [5-(9,9-difluoro-7- {2-[(1R,3S,4S)-2-{(2S)-2-[(methoxycarbonyl)amino]-3-methylbutanoyl}-2-azabicyclo [Pure Samples] hept-3-yl]-1H-benzimidazol-6-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-azaspiro [2.4] hept-5-yl}-3-methyl-1-oxobutan-2-yl] carbamate. It has a molecular formula of C49H54F2N8O6 and a molecular weight of 889.00 (Figure 1). Ledipasvir is a white to tinted (off-white, tan, yellow, orange, or pink), slightly hygroscopic crystalline solid. Ledipasvir is practically insoluble in water (<0.1 mg/mL) across the pH range of 3.0-7.5 and is slightly soluble below pH 2.3 (1.1 mg/mL). The partition coefficient (log P) for ledipasvir is 3.8 and the pKa1 is 4.0 and pKa2 is 5.0.5 Ledipasvir is an HCV inhibitor targeting the HCV NS5A protein, which is essential for both RNA replication and the assembly of HCV virions. Biochemical confirmation of NS5A inhibition of ledipasvir is not currently possible as NS5A has no enzymatic function. In vitro resistance selection and cross-resistance studies indicate ledipasvir targets NS5A as its mode of action.8
The combination of these two drugs is not official in any pharmacopoeia.11,12 Recently, a limited number of methods have been developed for the individual and simultaneous determination of both drugs. The degradation products of SOF under several stress conditions have been determined by HPLC.13,14 SOF's disposition was characterized into various in vivo cell types.15 Sofosbuvir in human plasma was determined by UPLC–MS/MS 232 method.16 SOF and its metabolite, GS-331007, in human plasma has been determined by UPLC-ESI–MS/MS method;17 ribavirin, sofosbuvir and its metabolite in rat plasma by UPLC-MS/MS.18 SOF in pure form,19 in bulk and tablet dosage form was determined by RP-HPLC.20
Finally, SOF was used as an internal standard (IS) in an UPLC-MS/MS method for the determination of daclatasvir (DAC) in human plasma21 and in mixture.22 While for LDV, only two methods have been published for its individual determination in bulk drug form by simple UV Spectrophotometry23 and by RP-HPLC24. Both Sofosbuvir and Ledipasvir in human plasma were determined by UPLC–MS/MS method25 besides some antiviral agents26. Ledipasvir, Sofosbuvir and its metabolite in rat plasma were also, determined by UPLC–MS/MS27 and HPLC methods.28–31 Recently RP-HPLC method and first-order derivative UV-spectroscopic method has been developed32 for the simultaneous determination of both SOF and LDV together.
The aim of this work is to reconsider spectrophotometry, being simple, popular and an essential bench tool in most laboratories, for the simultaneous determination of two important drugs indicated for the treatment of chronic HCV compared with reported sophisticated methods. Such determination of both drugs with high sensitivity, selectivity that is required to forensic pharmacy which is an application of the sciences of drugs to legal issues. A forensic pharmacist can be a valuable resource in legal cases relating to malpractice, adverse drug reactions, drunk and drugged driving, health care fraud, poisoning, and numerous other types of civil and criminal cases.33 Forensic science can e.g., reveal old alcohol dependence (AD), the internal comorbidities and the presence of any psychiatric comorbidity. Liver problems are one of the most common causes of alcohol-related liver damage. 45% of deaths from cirrhosis are alcohol-related.34
Instrumentation
UV- 1800 double beam UV-Visible spectrophotometer (Shimadzu-Japan) with highest resolution which Spectral bandwidth is (1nm from 190-1100 nm range) is used for all absorbance measurements with matched 1 cm quartz cells. Perform data analysis by software (UV-Probe 2.5.2).
Chemicals and reagents
Pure samples: Pure samples of sofosbuvir and ledipasvir were kindly supplied by the Egyptian Pharmaceutical and Chemical Industry (EPCI) Pharmaceutical Company part of HIKMA group, Beni-Suef, Egypt with claimed purity of 99.8% according to manufacturer certificates of analysis.
Pharmaceutical formulation: HETEROSOFIR PLUS (HARVONI®) 400mg/90mg 28 FCT (Batch No. 141610A) were manufactured by PHARMED Healthcare, 7th industrial zone, Al Sadat city, Al Menufia - Egypt. Each tablet is claimed to contain 400mg of Sofosbuvir & 90mg ledipasvir.
Solvent: Methanol of HPLC-grade is used as a solvent. It is obtained from (scharlau, Spain).
Preparation of standard solutions
Stock solutions of sofosbuvir and ledipasvir (1000μg /mL): Accurately weigh 100mg of each of SOF & LDV, transfer into 100mL volumetric flask, add 70mL of the solvent and sonicate to dissolve and complete to the mark with the same solvent and mix well.
Working standard solutions of sofosbuvir and ledipasvir (100μg/mL): Accurately transfer 10mL of SOF & LDV from their stock solutions in two 100mL volumetric flasks, add 70mL of the solvent and sonicate to dissolve and complete to the mark with the same solvent and mix well.
Laboratory prepared mixtures: Prepare a mixture of SOF and LDV containing different ratios from their working standard solutions (100μg/mL) into a series of 10mL volumetric flasks. Complete to the mark with the same solvent. The concentration of SOF and LDV were calculated from their corresponding regression equations under linearity for each method.
Application on pharmaceutical formulation (HETEROSOFIR PLUS 400mg/90mg 28 FCT): Weigh not less than 10 tablets and determine the average weight of one tablet. Grind to fine powder. Transfer an accurately weighed portion of the powdered tablets equivalent to the average weight of one tablet into 1000-mL volumetric flask. Add about 700mL of the diluent, sonicate till dissolve and complete to volume with the same diluent and mix well. Transfer an aliquot of 10mL from the lastly prepared flask into 100mL volumetric flask and complete the volume to the mark with the diluent to obtain a concentration of SOF & LDV (40 & 9μg/mL), respectively. The concentration of SOF and LDV in tablets was determined by spiking each of them by known concentration of pure standard drug then the spiked amount is subtracted. The validity of each of the aforementioned method was further confirmed by applying the standard addition technique.
Construction of calibration curves
Different aliquots of SOF and LDV equivalent to 5-50µg/mL, respectively, are separately transferred from their respective working standard solutions (100µg/mL) into two separate series of 100mL volumetric flasks and volume is completed to the mark with methanol and mixed well. Zero order absorption spectra of SOF and LDV are scanned in the range 200-400nm (Figure 2) and stored. The calibration curves relating the obtained absorbances to the corresponding concentrations are constructed and the regression equations are calculated. Calibration curves are constructed relating the first derivative of the absorbance of SOF at 274nm, versus the corresponding concentrations. The regression equations are computed.
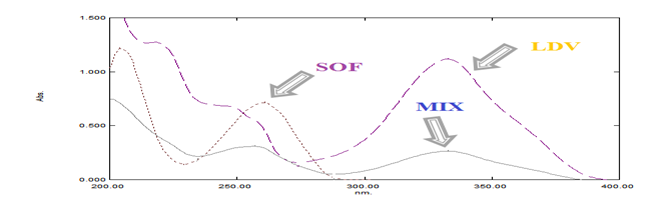
Figure 2 Zero order absorption spectra of 40µg/mL of each of SOF (….) and LDV (----) and mixture of 10 µg/mL of each of SOF and LDV (ــــــ) using methanol as a blank.
Direct spectrophotometric method (D0): In zero order absorption spectra, LDV is directly determined using its extended spectra at its λmax at 333nm, the calibration curve relating the obtained absorbances and the corresponding concentrations in the range 5-50µg/mL are constructed and the regression equations are calculated.
First derivative method (1D): First derivative spectrum of SOF is obtained using Δλ=10 and scaling factor =10. Peak amplitude values are recorded at a wavelength equals to 274nm versus the corresponding concentrations in the range 5 -50µg/mL and the regression equations are computed.
Ratio difference method: The scanned spectra of authentic mixtures are divided by the absorption spectrum of LDV (10μg/mL). The ratio spectra are recorded. Calibration curves are constructed by plotting the difference between the amplitude ratio difference of the SOF spectra at 270 and 208nm, versus the corresponding concentrations and the regression equations are computed.
Ratio subtracting method: Ratio subtracting method (RSM) plays an important role in solving the overlapping spectra of two drugs. The stored spectra of binary mixtures of SOF and LDV are divided by the absorption spectrum of 25μg/mL LDV as a dvisor then subtract constant value at the plateau region 290–375nm, therefore the zero-order absorption spectrum of SOF is obtained at 261nm by multiplying the obtained curve after subtraction by 25µg/mL of LDV as a divisor. Calibration curves relating the absorbance of zero order spectra of SOF at 261nm versus their corresponding concentrations are constructed and the regression equations are computed.
The purpose of this work is the development of facile, simple, sensitive, accurate and precise available spectrophotometric methods for the determination of binary mixture of SOF and LDV in their pure form and in pharmaceutical formulations. To determine the concentration, recovery for authentic mixtures and to validate each of the proposed methods the standard addition technique was applied. LDV can be easily determined by zero order (D0) spectrophotometry at λmax equals to 333nm as shown in Figure 2 without interference. On the other hand, SOF couldn’t be simply determined due to the overlapping of spectra, hence, the search and application for other alternative spectrophotometric techniques for SOF determination side by side with LED. First derivative (1D), ratio difference (RD) and ratio subtraction (RS) methods proved to be successful for the achievement of this target. During the preparation of the present work for publication, two spectrophotometric methods35,36 have been published. Although the aim of these methods, and ours, is the spectrophotometric determination of SOF and LED, however the approach of each method towards the achievement of this target is significantly different. These differences are focused in Table 1.
|
Parameter |
Present Method |
|
|
Nada, et al.2016 |
|
|
|
|
|
|
|
Abo-Talib, et al.2017 |
|
|
|||
|
SOF |
LDV |
SOF |
LDV |
SOF |
LDV |
||||||||||||
|
Method |
1D |
RD |
RS |
D0 |
λiso |
AS |
RS |
AUCC |
D0 |
AS |
RS |
AUCC |
3D |
1DD |
RD |
RS |
D0 |
|
Wave length |
274 nm |
270-208 nm |
261 nm |
333 nm |
262.4 nm |
262.4 nm |
262 nm |
245-265 nm |
325 nm |
262.4 nm |
325 nm |
315-335 nm |
281 nm |
265.8 nm |
270-250 nm |
261 nm |
333 nm |
|
Ranges (µg/mL) |
50-5 |
May-50 |
Feb-50 |
25-Feb |
May-35 |
14-Apr |
|||||||||||
|
Correlation coefficient |
0.9997 |
0.9993 |
0.9994 |
0.9997 |
- |
- |
0.9999 |
0.9999 |
0.9998 |
0.9999 |
0.9998 |
0.9998 |
0.9997 |
0.999 |
0.999 |
0.999 |
0.999 |
|
Accuracy (Mean) |
100.07% |
100.08% |
99.71% |
99.61% |
99.61% |
99.67% |
99.61% |
99.95% |
100.85% |
99.87% |
98.62% |
100.49% |
99.95% |
100.49% |
98.13% |
98.81% |
|
|
Advisor |
- |
10 |
25 |
- |
- |
- |
10 |
- |
- |
- |
- |
- |
- |
5 |
5 |
5 |
|
|
LDV(µg/mL) |
Done |
Not Done |
Done |
||||||||||||||
|
Statistical comparison |
Done |
Not Done |
Done |
||||||||||||||
Table 1 Comparison among the present methods and the newly published spectrophotometric ones for the determination of SOF and LDV in mixture
Very recently Four multivariate chemometric methods have been developed for the simultaneous determination of sofosbuvir and ledipasvir in their pure and pharmaceutical dosage forms37,38 where the zero-order absorption spectra of the prepared mixtures have been recorded over the wavelength range 200–400nm with 1nm interval. The obtained absorbance and concentration data matrix have been utilized to obtain calibration or regression analysis data which has been used for the prediction of the unknown concentrations of each drug in their mixtures. For convenience, each of the proposed methods will be discussed separately.
Methods development and optimization
Selection of the solvents, divisor and wavelengths is very important for method adjustment and development, so different solvents were tried such as distilled water, 0.01M HCl, 0.01M NaOH and methanol. Methanol proved to be the best solvent, which is used in the development of the most resolved spectra with minimum noise and maximum sensitivity. Also, selection of the divisor at the concentrations of 10 and 25µg/mL of LDV in the RD and RS, gave good selectivity and recovery. Different wavelengths were tried for the determination of SOF and LDV, respectively. That of 333nm for LDV and 274nm for SOF are found to be appropriate in the 1D; similarly, the difference between the amplitudes at the two wavelengths 208 and 270nm for SOF was applied in the RD. Finally, 261 nm for SOF in the RS gave good selectivity regarding laboratory prepared mixtures.
First derivative method
In this method, SOF could be determined by the first derivative spectrophotometric method using Δλ=10nm and scaling factor =10. Peak amplitude values are measured at a specific wavelength 274nm only, but it couldn't be determined at the wavelengths 245nm and 214nm due to overlapping (Figure 3). The calibration curve is constructed relating the first derivative absorbances of SOF at 274nm, versus the corresponding concentrations from which the regression equations are computed. Good correlation coefficient 0.9997, besides accuracy and recovery for both the authentic mixture and tablet dosage form at the specific wavelength 274nm are obtained.
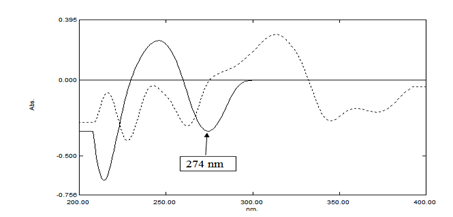
Figure 3 First derivative spectra of 40µg/mL of each of SOF (----) and LDV (ــــــــ) using methanol HPLC grade as a blank.
Ratio difference method
A suitable method for the determination of both SOF and LDV in their pure and dosage forms and also capable of solving overlapped spectra. The important role of the ratio difference spectrophotometric method (RD) is measuring the values of ratio difference at two wavelengths without the need of application of the derivative technique. The overlapped spectra of SOF at the two wavelengths 270 and 208nm, which gave values while the ratio difference of LDV is constant. All concentrations of SOF in the range 5-50μg/mL are divided by the spectrum of LDV (10μg/mL) (Figure 4). Absorbance values of ratio difference of the spectra of SOF at 270 and 208nm are plotted versus the corresponding concentrations where the calibration curve is obtained and the regression equations are computed.
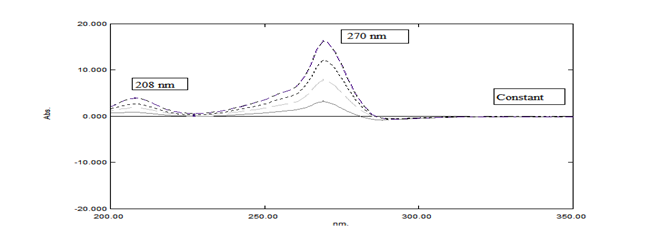
Figure 4 Ratio spectra of (5-50µg/mL) of SOF using the spectrum of 10µg/mL of LDV as a divisor showing the two selected wavelengths (270nm and 208nm).
Ratio subtraction method
The spectrum of the prepared mixture is divided by suitable concentration from the two drugs as a divisor. The result from division process gives a new spectrum. The constant is determined from the plateau region (290-375nm), then subtract the value of the constant from the ratio spectra. The multiply the obtained spectrum by the divisor. Finally, we can obtain a new zero order spectrum. This method is applied for SOF by dividing the prepared mixture by 25μg/mL of LDV as a divisor, subtract the constant in the plateau region (290-375nm) from ratio spectra followed by multiplying the new curve by the divisor. A new zero order SOF spectrum is obtained (Figure 5). Calibration curves are constructed by plotting the zero order absorbance values of SOF at 261nm versus the corresponding concentrations and the regression equations are computed.
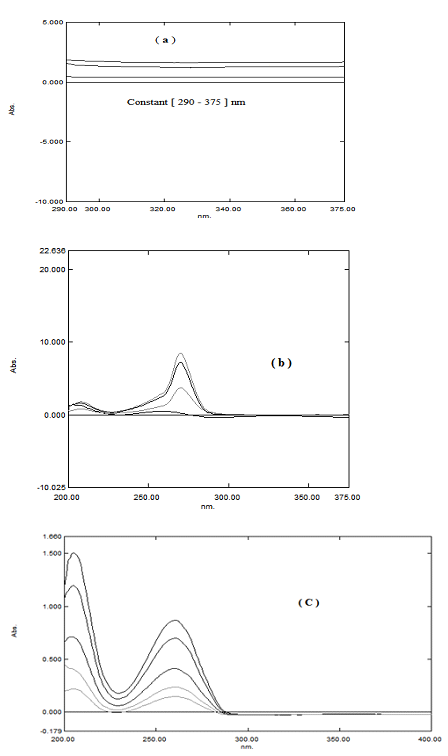
Figure 5 (A) Ratio spectra of mixtures of SOF and LDV using 25μg/mL of LDV as a divisor, (B) Ratio spectra after subtraction of constant, (C) new spectrum of SOF in the zero order.
Method validation
Each method was validated, in accordance with ICH guidelines.39
Linearity and range: The linearity of the proposed methods was obtained in the concentration range 5–50μg/mL for sofosbuvir and ledipasvir. Calibration graphs are plotted on the basis of the analysis of each calibration solution. The obtained results of correlation coefficients, slope and intercept indicate good linearity. Linearity results are shown in Table 2.
|
Parameter |
SOF |
LDV |
||
|
Method |
1D |
RD |
RS |
D0 |
|
Wave lengths |
274 nm |
(270 - 208) nm |
261 nm |
333 nm |
|
Range (µg/mL) |
5-50 |
5-50 |
5-50 |
5-50 |
|
Slope |
0.0085 |
0.3212 |
0.013 |
0.0288 |
|
Intercept |
-0.0024 |
-0.4057 |
-0.0154 |
-0.0101 |
|
Correlation coefficient |
0.9997 |
0.9993 |
0.9994 |
0.9997 |
|
Repeatability |
0.36 |
0.11 |
0.34 |
0.25 |
|
LODa (µg/mL) |
0.87 |
1.3 |
1.19 |
0.91 |
|
LOQa (µg/mL) |
2.63 |
3.94 |
3.62 |
2.76 |
|
*RSD%b |
0.139 |
0.338 |
0.402 |
0.556 |
|
*RSD%c |
0.582 |
0.688 |
0.81 |
0.945 |
Table 2 Regression and validation parameters of the proposed methods for determination of SOF and LDV
Precision
Repeatability: Expresses within-laboratories variations: The intraday RSD% (n=3), average of three concentrations (10, 15 and 30μg/mL) for SOF and LDV respectively were repeated for three times within the day. Good results are obtained as shown in Table 2.
Intermediate precision: The interday RSD% (n=3), average of three concentrations (10, 15 and 30μg/mL) for SOF and LDV, respectively are repeated three times in three successive days. Good results are obtained and presented in Table 2.
Detection and quantitation limits: These approaches are based on the Standard Deviation of the Response and the Slope. A specific calibration curve should be studied using samples containing an analyte in the ranges of LOD and LOQ. The residual standard deviation of a regression line or the standard deviation of y-intercepts of regression lines may be used as the standard deviation. LOD=3.3×σ/slope and LOQ=10×σ/slope, where σ=the standard deviation of the response Table 2.
Accuracy and recovery: Accuracy of the proposed methods is calculated as the percentage recoveries of pure samples of the studied drugs. Accuracy is assessed using three different concentrations covering the specified range (i.e. three concentrations and three replicates). Concentrations are calculated from the corresponding regression equations. The mean % recoveries for each of SOF and LDV are between 98.0% to 102% as shown in Table 3. The proposed methods are also applied for the determination of SOF and LDV in HETEROSOFIR PLUS (HARVONI®) 400mg/90mg 28 FCT. Good results are obtained (Table 4). The validity of the methods was also evaluated by applying the standard addition technique which also gives good accuracy and recovery (Table 4).
|
Sofosbuvir Standard Solution (µg/mL) |
Add μg/mL |
SOF |
Ledipasvir Spiked level (µg/mL) |
LDV |
|||||||
|
1D |
RD |
RS |
D0 |
||||||||
|
μg/mL (taken) |
μg/mL (found) |
Recovery% |
μg/mL (found) |
Recovery% |
μg/mL (found) |
Recovery% |
μg/mL (taken) |
μg/mL (found) |
Recovery% |
||
|
5 |
5 |
4.98 |
99.78% |
4.9 |
98.12% |
5.03 |
100.61 |
10 |
10 |
10.17 |
101.99% |
|
5 |
4.93 |
98.60% |
4.92 |
98.43% |
5.04 |
100.92 |
10 |
10.14 |
101.79% |
||
|
5 |
4.96 |
99.31% |
4.93 |
98.61% |
4.95 |
99.07 |
10 |
10.1 |
101.09% |
||
|
15 |
15 |
15.1 |
100.66% |
14.83 |
98.92% |
14.79 |
98.62 |
25 |
25 |
24.55 |
98.23% |
|
15 |
15.14 |
100.98% |
14.8 |
98.71% |
14.95 |
99.65 |
25 |
24.59 |
98.37% |
||
|
15 |
15.21 |
101.45% |
14.82 |
98.81% |
15.02 |
100.16 |
25 |
24.62 |
98.50% |
||
|
30 |
30 |
29.91 |
99.71% |
29.9 |
99.68% |
30.39 |
101.33 |
30 |
30 |
29.76 |
99.22% |
|
30 |
30.03 |
100.10% |
29.91 |
99.69% |
30.01 |
100.05 |
30 |
29.8 |
99.34% |
||
|
30 |
30.03 |
100.10% |
29.92 |
99.74% |
30.09 |
100.3 |
30 |
29.83 |
99.45% |
||
|
Accuracy (Mean) |
100.08% |
98.97% |
100.07% |
Accuracy (Mean) |
99.71% |
||||||
Table 3 Data of Accuracy for SOF and LDV.
aLimit of detection (3.3×σ/Slope) and limit of quantitation (10×σ /Slope).
*RSD%b & *RSD%c: the intra-day and inter-day respectively (n=3) relative standard deviation of concentrations (10, 15, 30µg/mL)
|
Pharmaceutical formulation |
Drug |
Method |
Found%a Mean±RSD |
Standard addition |
|
|
|
HARVONI 400/ 90FCT SOF, 400 mg(claimed) LDV, 90 mg (claimed) |
LDV |
[D0] λ= 333nm |
100.75±0.54 |
Added μg/mL |
Found μg/mL |
Recovery%b |
|
5 |
5.04 |
100.8 |
||||
|
10 |
10.11 |
101.11 |
||||
|
20 |
19.68 |
98.42 |
||||
|
Mean±RSD |
100.11±1.46 |
|||||
|
SOF |
[1D] λ=274nm |
100.60±0.65 |
5 |
10.06 |
100.66 |
|
|
10 |
4.97 |
99.58 |
||||
|
20 |
20.26 |
101.31 |
||||
|
Mean±RSD |
100.51±0.86 |
|||||
|
[RD] (270–208nm) |
100.93 ± 0.05 |
5 |
4.99 |
99.93 |
||
|
10 |
9.97 |
99.76 |
||||
|
20 |
19.9 |
99.54 |
||||
|
Mean±RSD |
99.74±0.19 |
|||||
|
[RS] λ=261nm |
100.46 ± 1.079 |
5 |
4.99 |
99.82 |
||
|
10 |
9.99 |
99.95 |
||||
|
20 |
20.28 |
101.41 |
||||
|
|
Mean±RSD |
100.40±0.88 |
||||
Table 4 Determination of SOF and LDV in their pharmaceutical formulation by the proposed methods and application of the standard addition technique.
aAverage of 6 determinations.
bAverage of 3 determinations.
Selectivity: The selectivity of the authentic mixture containing both drugs in different ratios within the linearity range is reported. It has been assessed by the analysis of the authentic mixtures containing different ratios of SOF and LDV which gave good accuracy and recovery for both drugs as shown in Table 5.
|
Ratio of SOF: LDV |
Added(µg/mL) LDV |
Added(µg/mL) SOF |
LDV |
SOF |
||
|
*Recovery % |
||||||
|
[D0] |
[1D] |
[RD] |
[RS] |
|||
|
λ= 333nm |
λ= 274nm |
(270 –208nm) |
λ= 261nm |
|||
|
1: |
10.00 |
20.00 |
101.79 |
101.95 |
101.6 |
101.55 |
|
4:5 |
40.00 |
50.00 |
99.33 |
100.97 |
100.8 |
99.96 |
|
2:3 |
30.00 |
45.00 |
101.54 |
99.13 |
99.05 |
100.77 |
|
3:4 |
30.00 |
40.00 |
101.07 |
100.35 |
101.33 |
98.93 |
|
2:9 |
10.00 |
45.00 |
100.05 |
99.39 |
99.63 |
100.08 |
|
8:7 |
40.00 |
35.00 |
101.59 |
101.92 |
101.79 |
100.82 |
|
1:1 |
50.00 |
50.00 |
101.55 |
100.03 |
101.1 |
100.48 |
|
Mean±RSD |
100.37±0.82 |
100.75±1.03 |
100.53±1.13 |
100.99±0.92 |
||
Table 5 Determination of SOF and LDV in laboratory prepared mixture by applying the proposed methods. *Average of three determinations
Statistical analysis: The results obtained for analysis of SOF and LDV by the proposed methods are statistically compared with those obtained by the HPLC method30 for the simultaneous determination of both drugs in a mixture. The results showed no significant differences among the proposed spectrophotometric methods and the published one as presented in Table 6. In order to compare the ability of the proposed methods for the determination of SOF and LDV, the obtained results are subjected to statistical analysis using one way ANOVA test. Results of ANOVA analysis showed no significant differences among the proposed spectrophotometric methods and the published method30 as presented in Table 7.
|
Method |
Reported Methoda 30 |
[D0] λ= 333 nm |
[1D] λ= 274 nm |
[RD] (270 –208 nm) |
[RS]λ= 261 nm |
|
|
LDV |
SOF |
LDV |
SOF |
SOF |
SOF |
|
|
Mean |
99.54 |
99.51 |
99.57 |
99.62 |
99.61 |
99.34 |
|
SD |
0.417 |
0.38 |
0.369 |
0.298 |
0.312 |
0.359 |
|
Variance |
0.174 |
0.145 |
0.136 |
0.089 |
0.097 |
0.129 |
|
N |
6 |
6 |
6 |
6 |
6 |
6 |
|
t-testb |
- |
- |
0.124 |
0.506 |
0.464 |
0.809 |
|
F-valueb |
- |
- |
1.275 |
1.628 |
1.494 |
1.117 |
Table 6 Statistical comparison between the results obtained by applying the proposed methods and the reported one for the analysis of SOF and LDV in their pharmaceutical formulation
*Values in parenthesis are corresponding to the theoretical values of t and F (p = 0.05).
|
Source of Variation |
||||||
|
SOF |
||||||
|
Between columns |
3 |
0.296 |
0.098 |
0.744 |
0.541 |
3.238 |
|
Within columns |
16 |
2.12 |
0.132 |
|||
|
Total |
19 |
14.205 |
|
|
|
|
|
LDV |
||||||
|
Between columns |
1 |
0.002 |
0.002 |
0.014 |
0.909 |
5.317 |
|
Within columns |
8 |
1.295 |
0.161 |
|||
|
Total |
9 |
1.297 |
||||
Table 7 One way ANOVA testing for the different proposed and the reported methods (mentioned in table 5) for the analysis of SOF and LDV in their pure and dosage forms
*There was no significance difference between the methods using one-way ANOVA at p < 0.05.
Novel spectrophotometric methods have been developed for the determination of sofosbuvir and ledipasvir in their tablet dosage form. They are simple, precise, specific, highly accurate, less time consuming for analysis, low cost and rapid. Ledipasvir can be determined in zero order, where there is no overlap. Sofosbuvir can be determined by three spectrophotometric methods viz., first derivative, ratio difference and ratio subtraction. They don’t require difficult equations or specific software. The developed methods can be easily applied for routine quality control analysis of both SOF and LDV in mixture.
This article does not contain any studies with human participants or animals performed by any of the authors. .
The authors are thankful to the Publishers of the Journal of Analytical & Pharmaceutical Research, MedCrave Group for the publication gift provided.
The authors declare that they have no conflict of interest. .

©2018 Hassouna, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.