Journal of
eISSN: 2473-0831


Research Article Volume 5 Issue 3
Correspondence: Ousman Deke Pierre Alexis, Department of Biomedical Sciences, Huazhong University of Science and Technology, China
Received: June 01, 2017 | Published: June 30, 2017
Citation: Alexis ODP, Guang Y, Guiaro MN (2017) New Approach for Skin Repair by Using Bacterial Cellulose Altered with Paraffin and Porous Bacterial Cellulose based Scaffold with Alginate. J Anal Pharm Res 5(3): 00141. DOI: 10.15406/japlr.2017.05.00141
Chronic wounds are difficult to heal due to several forms of infections. This issue can be addressed through the utilization of advanced approaches such as regenerative medicine and tissue engineering. One possible strategy could be the modification of bacterial cellulose (BC) structure to increase the current efforts of building scaffolds for tissue engineering. The current study was aimed to make structural comparative analysis of paraffin-altered BC and synthesis of its composite with alginate. Porous BC was produced through the addition of paraffin particles. Thereafter, a three dimensional scaffold of porous BC with alginate (BC/AL) was fabricated through the blending of BC and AL solutions. The paraffin particles were incorporated in the process of culturing Acetobacter xylinum for BC pellicle formation. Structural features of porous BC and BC/AL scaffold paralleled with original BC were investigated by the scanning electron microscope (SEM). SEM analysis revealed that porous BC possessed large surface (micro-scale pores) while the BC/AL scaffolds demonstrated a well-connected porous network. The purity and chemical structure of surfactants (Span 80 and OP-10) treated BC/AL scaffold was determined by Fourier transform infrared spectrum (FTIR) which confirmed the presence of any impurity in the form of paraffin in the 3D network of scaffold. Average water absorption analysis showed that pristine BC, porous BC, and BC/AL scaffold possessed 99.62%, 97.61%, and 96.94% water, respectively. Mechanical characteristics analysis of pristine BC, porous BC, Al, and BC/AL showed that these were conserved at certain levels between BC and AL. The MTT assay using RPMI 1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Gibco, USA) were used for human fetal hepatocyte L-02 cell line (Tongji Hospital, Wuhan, China) culture. Cells were incubated at 37oC, 5% CO2 and humid atmosphere. The scaffold developed in the current study can serve as possible material for the treatment of burns and chronic wounds treatment.
Keywords: bacterial cellulose, alginate, fabrication, paraffin, scaffold, biocompatibility, biomedical applications
Bacterial Cellulose (BC), produced by Acetobacter xylinum, consists of a biogenous nanofiber grating structure made by self-assembling in an efficient approach. Paralleled with other natural biodegradable polymers such as chitin, collagen and gelatin, BC offers much higher mechanical characteristics, which are needed in most situations when exploited as scaffold in tissue engineering. Some applications for BC in medical areas include artificial blood vessels for microsurgery,1 artificial skin for humans with extensive burns,2 scaffolds for tissue engineering of cartilage,3 and wound-dressing.4 Bacterial Cellulose expresses great hydrophilicity, non-allergenic, good absorption of liquids, and can be securely purified without any alteration of its characteristics or properties. BC itself has no antimicrobial action and function to prevent wound’s infection and also has Nano pores that cannot allow certain sizes of cells to get inside. This is why the addition of another polymer such as the Alginate on its blanket, play an important role in the improvement of its properties.
Alginate (AL), like BC, has been known for its applications in several fields in the biomedical sector. It is known for its absorption of exude anti-fungal, antimicrobial and wound healing properties. AL is useful in the wound management to reduce scar tissue. Alginate is useful for several applications as a biomaterial and especially as the supporting matrix or delivery system for tissue repair and regeneration. The outstanding properties of Alginate such as its biodegradability, non-antigenicity, biocompatibility, and chelating ability, have popularized its use in a variety of biomedical applications including drug delivery, tissue engineering and in some formulations preventing gastric reflux.5,6
Chronic wounds are difficult to heal because of a number of factors. infection, tobacco, cancer, malnutrition, diabetes, medications, deficiency of growth factor, the decrease of oxygen, the cells degeneration or inability to respond to growth factor, the slow cells proliferation and the absence of the support of the cells. Some of those need a surgical debridement or a foreign material in the depths of the wound. All these are the main hindrances to the wound healing process and remain to be the primary challenges to regenerative medicine. So it is important to use techniques of the regenerative medicine and tissue engineering to solve this problem. In this study, composites of alginate and bacterial cellulose whose structure (porosity) has been transformed by paraffin has been used. Especially in the developing countries; many chronic wounds do not heal because of inadequate wound care. Without proper care, the wound becomes covered with dead (necrotic) tissue. To achieve wound closure, all necrotic tissue must be removed (debrided), either with wet-to-dry dressings or with surgical debridement or tissue Engineering. Based on the physiological processes involved in wound healing, what is the impact of paraffin-altered BC and BC/AL composites on the treatment of burns and chronic wounds? Due to the composition and properties, the mixing of AL and BC has a positive impact on the healing of chronic wounds or the regeneration of the skin on the major burns? This study aimed at preparing porous BC and fabricating its scaffold with BC/Alginate for a potential biomedical application which uses the synergic beneficial aspects of both polymers. The main objectives of this study were, the preparation of paraffin particles; the culture of Acetobacter xylinum in order to synthesize the BC with paraffin particles; the comparison of the SEM images of the modified and the normal BC pores and the final one was to make BC/Alginate scaffold.
Materials
The reagents used in this work included: glucose, peptone (OXOID LTD, BASINGSTOKE, HAMPHIRE, ENGLAND), yeast extract, acetic acid, paraffin wax, surfactants: Span 80 and OP-10, series Q/CYDZ 144-2013 (Sinopharm Chemical Reagent Co…Ltd, China), disodium phosphate (Acros, Biochemical), deionized water (Aqoapro CO., Ltd, Chong-qing, China), and sodium hydroxide (Acros, Biochemical, China) etc. All chemicals were used as received without further purification.
Preparation of paraffin particles
Ten grams (10g) of polyvinyl alcohol (PVA) was dissolved in 800mL of distilled water to a boil scope for at least 90 minutes, and then the solution obtained was introduced in a water bath at 80oC while shaking the solution with a magnetic stirrer set at 30-35 revolution per minute. Then two paraffin blocks (approximately 30g) were dissolved by gentle heating in a small beaker and then introduced into the PVA solution gently. The water solution about 3oC ice prepared in advance was added to the mixture at once to rapidly lower the temperature and allow the formation of micro particle paraffin, which were introduced in a sieve with pores of different sizes (1.0mm; 0.5mm; 0.3mm), then washed in tap water. The individual particles obtained were dried and were subsequently introduced into alcohol solutions of 75% contained in beakers to sterilize them for 1 hour. Then they were washed with distilled sterilized water to remove alcohol and store until use.
Preparation of the microbial cell culture
The culture medium was prepared according to standard Schramm & Hestrin: 6.8g of disodium hydrogen phosphate dotecahydrate; 5 g of yeast extract; 5 g of Peptone; 1.5 g of citric acid monohydrate and finally 20 g of glucose, all homogenized in 1 liter of distilled water. The resulting solution was poured into a glass bottle of 1 liter of volume was then sterilized for 20 minutes at 121oC.
Production of the porous BC and fabrication of the BC/AL scaffold
Culture begins with the introduction of paraffin particles into the different Erlenmeyer, until complete coverage of the bottom. All this with great caution so as not to contaminate the environment. By following the culture solution is add. Furthermore to ensure a good connection paraffin particles, the medium is slightly heated in a water bath of about 38oC. 5ml from a solution containing Acetobacter Xylinum is added to each Erlenmeyer flasks which are then well covered and introduced into the culture chamber set at body temperature for a period of three weeks depending on the particle size paraffin who limit the mobility of Acetobacter xylinum.
Fourier transform infrared spectroscopy (FTIR) of pristine BC, porous BC, and BC/AL scaffold
FTIR spectra of the freeze-dried pristine BC, porous BC, and BC/AL scaffold samples were recorded using a Perkin Elmer FTIR spectrophotometer [Spectrum GX &Auto image, USA; spectral range: 4000-400cm−1; beam splitter: Ge-coated on KBr; detector: DTGS; resolution: 0.25cm−1 (step selectable)]. For analysis, the samples were mixed with KBr (IR grade, Merck, Germany) pellets and processed further to obtain IR data, which were transferred to a computer to acquire the spectra.
Scanning electron microscope (SEM) of pristine BC, porous BC, and BC/AL scaffold
The morphological characteristics of freeze-dried pristine BC, porous BC, and BC/AL scaffold samples were investigated through FE-SEM analysis. Briefly, all samples were prepared separately on a brass holder and coated with osmium tetroxide (OsO4) using a VD HPC-ISW osmium coater (Tokyo, Japan) and analyzed through a Hitachi S-4800 & EDX-350 (Horiba) FE-SEM (Tokyo, Japan).
MTT assay
RPMI 1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Gibco, USA) were used for human fetal hepatocyte L-02 cell line (Tongji Hospital, Wuhan, China) culture. Cells were incubated at 37oC,5% CO2 and humid atmosphere. According to ISO 10993-5 (an international standard), the samples were sterilized by autoclaving and then added to the cell culture medium with a final concentration of 2 mg/ml. The samples were placed in 37oC at least for 72h and then centrifuged at room temperature for 10mins to get supernatant. The extracts (0.22um) were removed with a filter then sterilize and place at 4oC-8oC until use. Following the manufacturer’s instruction, the cells were seeded in 96-well plates before the day of the CCK-8 assay for overnight to make the cells at the mid-log phase of growth. The old culture medium were changed to the extract of samples at 100ul pre well, using the culture medium itself as negative control and non-cell with medium as blank control. After incubating for 24h, 48h, 72h, the cells were treated with 10ul/well cck-8 (Dojindo, Japan), and shacked for 30mins then incubated further for 1.5h at 37oC in 5% CO2 atmosphere. Every sample had 4 repeats and the absorbance values were read in triplicate with a wavelength of 450nm (Multiskan FC Microplate Reader, Thermo Fisher, USA).
FTIR analysis of pristine BC, porous BC, and BC/AL scaffold
FTIR spectra of BC in addition for paraffin can be observed in Figures 1 & 2. Normal cellulose has certain point peaks all-inclusive 1600, 2900 and 3400cm-1, but paraffin has property peaks around 720, 1450 and 2900cm-1. By getting spectra of cleaned BC and matching them with the spectra for pure BC/paraffin, deduction can be illustrated about the chemical composition of the material. The blue spectra represent the purification surfactant Span 80 and OP-10 (Figure 2). The black spectrum shown is the sample of interest. The different peaks: 814, 82; 1025, 98; 1408, 14; 1593, 77; 2929, 29 and 3342, 55 observed in the porous BC samples compare to the surfactant spectra, indicating that no residues of paraffin is left within it. But also these two spectra are similar.
Morphological characteristics of pristine BC, porous BC, and BC/AL scaffold
Morphological characteristics of BC: The (MA) matrix architecture observed in Figure 3 means the way in which a wholesale material appears in space, at the macroscale and nanoscale (i.e., tissue-length scales, cellular, molecular, and respectively). The MA expresses the mechanical assembly of the scaffold; it also explains the primary void space that is obtainable for connection of progenitor’s tissue to shape new tissue, involving new blood vessels, in addition to the pathways for convection and diffusion (mass transport). Normally, most of the scaffolds are considered to have an interior porous assembly of hole spaces that are communicated via pores or networks on the scale. Generally, larger pores support deeper penetration or infiltration of new tissues. This is appropriate to the current clinical procedure of supplying large cavities with particulate materials, as the hole spaces between arranged particles are mostly an order of significance larger than the known microstructure or hole dimension of most granules themselves. So increasing the pores of biomaterial for tissue engineering could offer the larger macrostructure needed for deeper revascularization and the new tissue can took place.

Figure 3 SEM of a bacterial cellulose sample showing a coherent 3-D appearance shaped by cellulose fibers linked by physical connection. 10.000x and 80.000x.
Morphological characteristics of AL, AL/BC and BC altered by paraffin: Nanostructure characteristics can also take the part of an important function in scaffold. These Nano pores are normally too small to affect where the cells can or cannot move, but may even have important results on cell performance by modifying surface texture and diffusion of resolvable materials. Every part of other structures being identical, the existence of connected Nano porosity inside the walls of a spongy or porosity characteristic can give the root of an abundant wider path for mass transport, in that way increasing cell differentiations in the scaffold (Figures 4 & 5). The Figure 5 represents a mixture of Alginate and BC, which gives it the appearance with interconnected pores and has spherical pores appearance. But the Figure 4 represents AL alone, and compare to Figure 5, its pores are not so spherical but are interconnected. In the Figure 6, the pores are spherical and interconnected. However, there are some particles of paraffin which still in the pores. These are presently being used to form or produce strategically adapted channels and pores or holes and expressed macroscopic appearances. The fabrication of more well-defined porous assemblies suggest the potential for better control throughout the distribution of wholesale material within an implant site in addition to control over configurations of cells migration, fluid movement, and dispersion through the design.
MTT assay
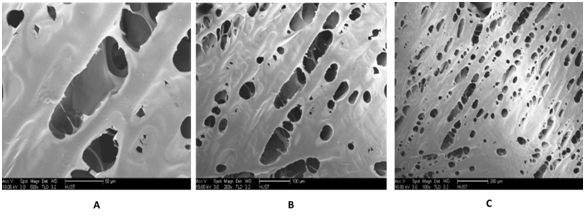
Figure 4 SEM of Alginate sample showing a coherent 3-D appearance shaped by interconnection of pores after lyophilization. A: 500x, B: 200 x and C: 100x.
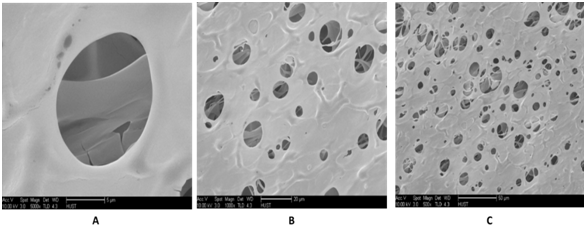
Figure 5 SEM of Alginate/BC sample showing a coherent 3-D appearance shaped by interconnection of pores after lyophilization of Alginate. A: 5000x, B: 1000x and C: 500x.
In the two cases of MTT essay (with control and without control), we observed the same phenomena during the variations of the cells used in BC, BC-al and BC-pore after 24 hours, 48 hours and 72, (P.value < 2.2e-16; CI=95%). This result means that the bovine cells used have a very good affinity and capacity to grow in the BC and BC-al, because the membrane is not toxic for the cells, which is not the case in the BC-pore certainly because of the fact that the pores created in the BC membrane with help of paraffin remain blocked by it in some places. Those which influence cells multiplication (Figure 7).
The chemical composition in models or samples can be explored using FTIR. This process applies mid-infrared rays (wavenumber between about 4,000 and 400cm-1) which are diffused within a sample, permitting the energy to be captivated in it, producing the chemical links inside the material to pulsate. The functional groups contained by the sample have an affinity to captivate in the same measurement lengthwise, apart from the structure of the entire molecule. Suitable to this characteristic, it’s probable to find functional groups in a sample and competing the spectra gained with spectra of identified materials. The chemical compositions in an unidentified sample can be observed by this way. One immense benefit of exploiting FTIR spectroscopy is that, it’s a quick technic to complete lots of data concerning the importance of the material.7 Additionally, the sample’s preparation is reasonably easy to do and the chemical analysis quite economical reasonable. The most important negative aspect of FTIR spectroscopy is the information about the atoms that it does not give, due to single atoms not having a few chemical bonds which can captivate rays and in that way begin to pulsate.7 According to the same intention, it’s not possible to identify homonuclear diatomic molecules and noble gases by using FTIR spectroscopy. In addition, FTIR spectroscopy is inadequate when it goes to exploring extremely complicated samples, for the reason that these will hold many functional groups, which cause the problem of interpretation during the analyses.
FTIR spectra of BC in addition for paraffin can be observed in Figure 1. Normal cellulose has certain point peaks all-inclusive 1600, 2900 and 3400cm-1, but paraffin has property peaks around 720, 1450 and 2900 cm-1. Via getting spectra of cleaned BC and matching them with the spectra for pure BC/ paraffin, deduction can be illustrated about the chemical composition of the material.
SEM is an analysis technical where the topography surface of materials or samples are explored by scanning them via a ray of high energy electrons, providing improvement to a comprehensive 3D image of the samples.8 This can be done on many heterogeneous materials in the collection of micrometer to nanometer scale. The notion of the procedure was firstly explained by Max Knoll in 1937. That technique exploits electrons which are emanated from a cathode (firstly made from tungsten, but now other materials such as LaB6 and gold are being used) of an electron gun, via using a high voltage.8 One great improvement with SEM is that is can be useful onto many materials, providing high resolution images of the most bulky materials.8 In addition, the method has a large extent of field and giving rise to the 3D appearance mentioned earlier. The obstacles with this procedure include that the preparations needed to make specimens electronically, accomplished as well as the preparations demanded to enable analysis of biological samples. Extra disadvantage is that only the top of the specimen is studied. According to the previous study, the structures of Bacterial Cellulose are shaped by extracellulary-emitted nanofibers made by different species of bacteria, namely Acetobacter xylinum, Agrobacterium, Rhizobium, and Sarcina.9 These nanofibers are approaching 100nm in diameter and their appearance or configuration show a coherent 3D grating. In the macroscopically appearance, the Bacterial Cellulose grating is fabricated as a pellicle that gets the shape of the container where the bacteria is developed.10 The nanofibers of Bacterial Cellulose are ribbon-like structures of around 100 µm in length and around 100nm in diameter and are fabricated of bundles of cellulose micro fibrils of 2 to 4nm as diameter.9-11 In the natural stage, Bacterial Cellulose is a water-swollen grating of nanofibers like showing in Figure the SEM image of natural Bacterial Cellulose network. At the same time, Grande et al.12 have exploited image analysis to gauge the morphological characteristic of dried Bacterial Cellulose gratings. The typical net dimension (distance relating junction points) is 0.523±0.273µm, whereas the direction (the typical angle created by the x-axis and the segments) of the nanofibers is 85.64±0.56°. In previous years, and even today, several studies have been conducted to find a solution in the healing process of wounds and burns based either cell cultures or manufacturing of natural organic compounds.
Possibilities for the structural model of tissue scaffolds are practically unlimited. The macro-assemblies embrace regular geometric forms (e.g., pellets, blocks and dowels), shapeless structures (e.g., granules, randomly packed chips or fibers), arbitrarily incorporated structures (e.g., freeze-dried materials of foams), and officially designed regular assemblies (e.g., printed, woven, machined or assembled structures). Gel’s preparations can additionally also be prepared from fibers or powders, via blending them with moldable agents (e.g., cellulose, glycerol and hyaluronan) or via operating in situ polymerization with use of photochemical, chemical or enzymatic technics.13 In certain circumstances, a needed structure has been copied from nature, like the vastly interconnected spongy structure of some corals or cancellous bone.14 The choosy processing (size, machining and density selection, demineralization and washing) provides a diversity of relatively improved materials for usage in particular clinical situations. Most of the methods for making porous scaffolds (i.e., freeze-drying, particulate leaching, phase separation and gas infusion) generate dispersed cavities and linking pores such as in a sponge by using particles or foams when the scaffold is congealed. Previously studies shown that, substantial improvements have been made in the techniques for creating more complete categorized of microstructures from different materials. These are presently being used to form or produce strategically adapted channels and pores or holes and expressed macroscopic appearances. The fabrication of more well-defined porous assemblies suggest the potential for better control throughout the distribution of wholesale material within an implant site in addition to control over configurations of cells migration, fluid movement, and dispersion through the design.15
Quantity of cell viability and multiplication shapes the basis for several in vitro tests of a cell population’s reaction to external influences. The diminution of tetrazolium salts is now commonly accepted as reliable way to observe cell growth. The 3, 4, 5-dimethylthiazolyl-2, 2, 5-diphenyltetrazolium bromide (yellow tetrazolium MTT) is reduced by metabolically active cells in the other part by the action of dehydrogenase enzymes, to create reducing equivalents such as NADPH and NADH. The result which known as intracellular purple formazan can be solubilized and counted by spectrophotometric methods.
The MTT Cell growth Assay, calculates the cell proliferation value and conversely, when metabolic events lead to necrosis or apoptosis, the diminution in cell viability. Several of tests steps have been minimalized as possible to accelerate sample managing. The MTT Reagent generates low background absorbance rates in the absence of cells. For each cell type the linear association between cell quantity and signal generated is founded and allowing a precise quantification of variation in the rate of cell division. RPMI 1640 medium (Hyclone, USA) supplemented with 10% fetal bovine serum (Gibco, USA) were used for human fetal hepatocyte L-02 cell line(Tongji Hospital, Wuhan, China) culture. Cells were incubated at 37oC with 5% CO2 and humid atmosphere.
In this study, porosity of Bacterial cellulose produced by Acetobacter xylinum was enhanced through the use of paraffin particles. The paraffin particles modified the bacterial cellulose pores giving it exceptional properties which can be used in the field of healing process of wounds and burns. The paraffin-altered BC can have a three dimensional structure, allowing it to be the cell support, accelerate the differentiation of cells. Its retention capacity allowing them to secrete extracellular matrix and contribute to the formation of new tissue in wound healing process. However the production of BC by incorporation of paraffin particles alters the structure of bacterial cellulose therefore requires much attention since it is responsible of many errors if precautions are not taken. Firstly, sieve pores are used to control and collect the desired size wax particles and it should not be done by hand. The sieve pores makes it possible to control the size of individual particles which are collected. Sterilization of paraffin particle gives reassurance that all alcohol used is well removed in order not to influence the culture medium. One of the most important things in this work is the choice of particles and the type of surfactant used. All of the particles must be removed by the surfactant used and well rinsed such that the final material contains no particles or surfactant. This was done in order to prevent the interference of the particles during the visualization of the enhanced BC with the SEM. Also the presence of surfactant in the bacterial cellulose prevents it from freezing and also allows its drying.
It was also noticed during the synthesis of the bacterial cellulose phase, when the small size particles for example 0.13mm and 0.3mm was added to the culture, the production of BC was not good, it gives a cob web appearance of the membrane formed which is fragile at the slightest pressure compared to when 0.5mm and 1.0mm particles size were used which gives a good bacterial cellulose appearance with large pores. This could be explained by the fact that the particles of smaller sizes do not provide enough space for bacteria to move and thereby connect the particles together with cellulose fibers that they secrete. And so it took too long to successfully synthesize such a membrane with small particle sizes. Porosity is a good property for bacterial cellulose used for wound healing.
Porous BC and BC/AL scaffold with a relatively high porosity were successfully fabricated and purified, using porogens as paraffin particles of varying pore diameter. We can rightly say that paraffin altered the structure of BC. Criteria for scaffold were set up after characterization using Scanning Electron Microscope (SEM) and it proved that paraffin improved porosity of BC. Additionally, the importance of interconnectivity between pores was not established due to the instrumental limitation used for cultivation BC. It can further be concluded that paraffin-altered BC and then the combination of BC with Alginate has potential to enhance wound and burn healing, because of their similarity structure appearance. Also in the literature, the biocompatibility, biodegradability and retention of BC and the properties of Alginate could probably accommodate and accelerate the process of division and the extracellular matrix of skin tissue secretion. Thus this is a huge step forward in the field of skin tissue regeneration. This study used the paraffin particles to modify the BC structure. The modification gave to the BC, a three dimensional appearance. The combination of BC/AL gave also a good material which can help in that area. Future research in this area should be done using Alginate with specific receptor combined with modified BC to help the cells to attach in the composite during the cells culture. Additionally, for the increasing of approval of the porous BC and BC/AL scaffold in the field of wounds or burns skin, the culture by using fibroblast should be done.
All authors contributed to the editing, designing, preparation and final review of the manuscript.
Authors thank the collaborators of their respective institutions for the comments on the manuscript.

©2017 Alexis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.