Journal of
eISSN: 2473-0831


Research Article Volume 7 Issue 2
Correspondence: Mansuri Reema, Department of Quality Assurance, Pioneer Pharmacy Degree College, Vadodara- 390019, Gujarat, India, Fax -9624099393
Received: March 04, 2018 | Published: April 13, 2018
Citation: Reema M, Dhara P, Khushboo P, et al. In depth investigation of quantitative analytical and bioanalytical techniques of hepatitic drug sofosbuvir in different matrices: a review. J Anal Pharm Res. 2018;7(2):206-220. DOI: 10.15406/japlr.2018.07.00228
Qualitative and quantitative estimation plays a key role in ensuring the safety and efficacy of drugs in different matrices. A detailed literature survey is one of the most essential requirements for all focused research activities. Sofosbuvir is the drug of choice for Hepatitis C virus infection with a high cure rate. It inhibits RNA polymerase enzyme which is responsible for replication of hepatitis C virus RNA. Sofosbuvir was approved by the U.S. Food and Drug Administration (FDA) in 2013. Sincere effort has been made in the present review to collate all the relevant literature published in various pharmaceutical journals for determination of sofosbuvir in different matrices both individually and in combination with other drugs. The review highlights the basic as well as advanced techniques performed for estimating sofosbuvir. Among different methods, HPLC and UV-Visible spectrophotometry are the most widely used techniques applied by the researchers. Detailed validation parameters are also given for the methods, which helps the researchers to select an analytical technique based on the information sought.
Keywords: sofosbuvir, analytical methods, estimation, matrices
CSA, chemical abstracts service; USP, united state pharmacopoiea; FDA, food and drug administration; ICH, international conference on harmonization; RP-HPLC, reverse phase high performance liquid chromatography; UHPLC, ultra high performance liquid chromatography; LC-MS-MS, liquid chromatography-mass spectrometry-mass spectroscopy; RP-UHPLC-DAD-MS, reversephase –ultrahigh performance liquid chromatography-diode array detector-mass spectroscopy; UHPLC-MS/MS, ultra high performance liquid chromatography-mass spectroscopy/mass spectroscopy; TLC, thin layer chromatography; UPLC-MS/MS, ultra performance liquid chromatography tandem mass spectrophotometry; CAN, acetonitrile; DMSO, dimethylsulfoxide; IR, infrared spectroscopy; SOFO, sofosbuvir; VELP, velpatasvir
Hepatitis C is a disease of the liver caused by the hepatitis C virus (HCV). The natural targets of HCV are hepatocytes and lymphocytes B in both acute Hepatitis C and chronic Hepatitis C. Virus lie in a dormant state until entering the living cell of host, where it will then hijack the cell’s hardware to replicate itself.1 RNA-dependent RNA polymerase, an enzyme critical in HCV replication, lacks proofreading capabilities and generates a large number of mutant viruses known as quasispecies. These represent minor molecular variations with only 1-2% nucleotide heterogeneity.2,3 Here, Mechanism of Hepatitis C shown in Figure 1.
Sofosbuvir (C22H29FN3O9P) is a new drug candidate for hepatitis C treatment. it is chemically known as Isopropyl (2S)-2-[[[(2R,3R,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyl-tetrahydrofuran-2-yl]methoxy-phenoxy-phosphoryl]amino]propanoate (Figure 2). Molecular weight of Sofosbuvir is 529.435g/mol. Physical properties and taxonomy are mentioned in Table 1–4, respectively.4 It is potent in inhibiting the HCV NS5B RNA-dependent RNA polymerase, which is responsible for viral replication. Being a nucleotide prodrug it undergoes intracellular metabolism to produce GS-461203, active uridine analog triphosphate and exhibits action by incorporation into HCV RNA by chain termination facilitated by NS5B polymerase. Since GS-461203 is not an inhibitor of human DNA and RNA polymerases nor an inhibitor of mitochondrial RNA polymerase it inhibits the polymerase activity of the NS5B from HCV genotype 1b, 2a, 3a and 4a.5
State |
White crystline solid |
Water solubility |
0.824mg/ml |
Pka |
9.3 |
Log P |
1.62 |
Melting point |
120◦C-125◦C |
Storage |
<30◦C |
Table 1 Physical properties7
Kingdom |
Organic compounds |
Super class |
Nucleosides, nucleotides, and analogues |
Class |
Pyrimidine nucleosides |
Subclass |
Pyrimidine 2'-deoxyribonucleosides |
Direct parent |
Pyrimidine 2'-deoxyribonucleosides |
Alternative parent |
Alpha amino acid esters/Alanine and derivatives/Phosphoric diester monoamides/Phenoxy compounds/ Pyrimidones/Hydropyrimidines/Organic phosphoramides/Vinylogous amides/Tetrahydrofurans/Heteroaromatic compounds |
Substitutents |
Pyrimidine 2'-deoxyribonucleoside/Alpha-amino acid ester/Alanine or derivatives/Alpha-amino acid or derivatives/Phenoxy compound/Phosphoric diester monoamide/Pyrimidone/Monocyclic benzene moiety/Benzenoid/Hydropyrimidine |
Molecular framework |
Aromatic heteromonocyclic compounds |
External descriptors |
organofluorine compound, ring assembly, L-alanyl ester, phosphoramidate ester, nucleotide conjugate (CHEBI:85083) |
Table 2 Taxonomy7
Bio availability |
92% |
Cmax |
567µg/ml |
Plasma protein binding |
61-65% |
Metabolism |
In Liver |
Half Life |
0.4 hours |
Excretion |
|
Table 3 Pharmacokinetics7
Generic name |
Brand name |
Dosage form |
Strength |
Manufacturer |
Sofosbuvir |
Sovaldi |
Tablet |
400mg |
Gilead Sciencies |
Sofosbuvir |
Hepcinat |
Tablet |
400mg |
Natco Pharma Ltd. |
Sofosbuvir |
Sofovir |
Tablet |
400mg |
Hetero Pharma Ltd. |
Sofosbuvir |
Sofab |
Tablet |
400mg |
Ranbaxy Pharma Ltd. |
Sofosbuvir |
E-Hep |
Tablet |
400mg |
Euphoria Pharma Ltd. |
Sofosbuvir |
Sovihep |
Tablet |
400mg |
Zydus Heptiza Ltd. |
Sofosbuvir |
MyHep |
Tablet |
400mg |
Mylan Pharma Ltd. |
Sofosbuvir |
Hepcvir |
Tablet |
400mg |
Cipla Pharma Ltd. |
Sofosbuvir |
Sofocure |
Tablet |
400mg |
Emcure Pharma Ltd. |
Sofosbuvir |
Novisof |
Tablet |
400mg |
Wockhardi |
Sofosbuvir |
Sofosbuvir |
Tablet |
400mg |
Zumeinnehmen Pharma Ltd. |
Sofosbuvir |
Virso |
Tablet |
400mg |
Strides Shasun Pharma Ltd. |
Sofosbuvir+Velpatasvir |
Velpanat |
Tablet |
400mg/100mg |
Natco Pharma Ltd. |
Sofosbuvir+Velpatasvir |
Resof |
Tablet |
400mg/100mg |
Dr.Reddy’s Lab. |
Sofosbuvir+Velpatasvir |
Velasof |
Tablet |
400mg/100mg |
Hetero Pharma Ltd. |
Sofosbuvir+Velpatasvir |
Sofosvel |
Tablet |
400mg/100mg |
Beacon Pharma Ltd. |
Sofosbuvir+Velpatasvir |
Epclusa |
Tablet |
400mg/100mg |
Gilead Pharma Ltd. |
Sofosbuvir+Velpatasvir |
Sovihep V |
Tablet |
400mg/100mg |
Zydus Heptiza Ltd. |
Sofosbuvir+Velpatasvir |
MyHep All |
Tablet |
400mg/100mg |
Mylan Pharma Ltd. |
Sofosbuvir+Velpatasvir |
Hepcvir |
Tablet |
400mg/100mg |
Cipla Pharma Ltd. |
Sofosbuvir+Velpatasvir |
Valpaclear |
Tablet |
400mg/100mg |
Abbott Pharma Ltd. |
Sofosbuvir+Ledipasvir |
Ledifos |
Tablet |
400mg/90mg |
Hetero Pharma Ltd. |
Sofosbuvir+Ledipasvir |
MyHepLVIR |
Tablet |
400mg/90mg |
Mylan Pharma Ltd. |
Sofosbuvir+Ledipasvir |
LediHep |
Tablet |
400mg/90mg |
Zydus Heptiza Ltd. |
Sofosbuvir+Ledipasvir |
Virpas |
Tablet |
400mg/90mg |
Strides Shasun Pharma Ltd. |
Sofosbuvir+Ledipasvir |
Cimvir-L |
Tablet |
400mg/90mg |
Biocon Pharma Ltd. |
Sofosbuvir+Ledipasvir |
Hepcvir L |
Tablet |
400mg/90mg |
Cipla Pharma Ltd. |
Sofosbuvir +Ledipasvir |
Resof-L |
Tablet |
400mg/90mg |
Dr’reddy’s Lab |
Sofosbuvir +Ledipasvir |
Harvoni |
Tablet |
400mg/90mg |
Gilead Sciences |
Sofosbuvir+Velpatasvir+Voxilaprevir |
Vosevi |
Tablet |
400mg/100mg/100mg |
Gilead Sciences |
Sofosbuvir metabolize in liver by pharmacologically active nucleoside analog triphosphate. Pharmacokinetic properties are mentioned in Table 5. Metabolism is activated by hydrolysis of carboxyl ester moiety in the presence of human cathepsin A (CatA) followed by phosphoramidate cleavage and phosphorylation by the pyrimidine nucleotide biosynthesis pathway. GS-331007 a nucleoside metabolite formed due to dephosphrylation lacks anti-HCV in vitro activity due to inefficient rephosphorylation.6
Parameters |
Result |
Linearity range (µg/ml) |
5-40 |
Correlation coefficient |
0.9996 |
%Recovery (%) |
98.33-101.76 |
Precision intraday (%RSD) |
0.48 |
Table 5 Validation parameters reported by Khedkar PM et al.16
The metabolites of Sofosbuvir are CatA- human cathepsin A; CES1- carboxyl esterase 1; Hint1 - histidine triad nucleotide-binding protein 1; NDPK – nucleoside diphosphate kinase; UMP–CMP kinase-uridine monophosphate–cytidine monophosphate. Patient who were taken Sofosbuvir in HCV genotype 1, 2, 3 or 4 infections and HCV/HIV-1 co-infection, suffered side effects like fatigue, headache, nausea, insomnia and anaemia. This all side effects occurs in hepatocellular carcinoma and severe renal impairment when sofosbuvir taken in combination with ribavirin and peginterferon alfa.7
History
Sofosbuvir was discovered in 2007 by Micheal Sofia, a scientist at Pharmasset and drug was first tested in people in 2010.8 2011 Gilead Sciences bought Pharmasset for about $11 billion.9 In Gilead submitted the new drug application for sofosbuvir in combination with ribavirin in April 2013 and in October 2013 it received the FDA,s Breakthrough therapy designation.10 In December 2013, the FDA approved Sofosbuvir in combination with ribavirin for oral dual therapy of HCV genotypes 2 and 3, and for triple therapy with injected pegylated interferon and RBV for treatment-naïve people with HCv genotype 1 and 4.11,12 Two months before, the FDA had approved another drug for Hep C, Simeprevir. In 2014 the fixed dose combination drug sofosbuvir/ledipasvir, the latter a viral NS5A inhibitor, was approved.13 Sofosbuvir/velpatavir was approved for medical use in the United States in 2016 (Figure 3).14
Clinical trials
The efficacy and safety of sofosbuvir in patients with different HCV genotypes and with various combinations of drugs have been tested in numerous clinical trials. A dose of 400mg of sofosbuvir has been found to be most effective, with treatment durations ranging from 12 to 24 weeks, in various combinations of PEG-IFN and ribavirin in phase 2 clinical trials. The NEUTRINO study found SVR to be 90% (95% CI, 87 to 93) 12 weeks after therapy with sofosbuvir + PEG-INF + ribavirin; this was found to be superior to the adjusted historical response rate of 60% (P<0.001). Similar positive results have been found in numerous phase 3 clinical trials. Furthermore, recent phase 1 and 2 studies of sofosbuvir in combination with other DAAs have also shown promising results (Figure 4).15
Analytical methods for estimation of sofosbuvir in bulk drug, pharmaceutical formulation and biological fluids
Many different analytical methods have been reported for the estimation of Sofosbuvir (SOF) in bulk and dosage form as well as in biological fluids.
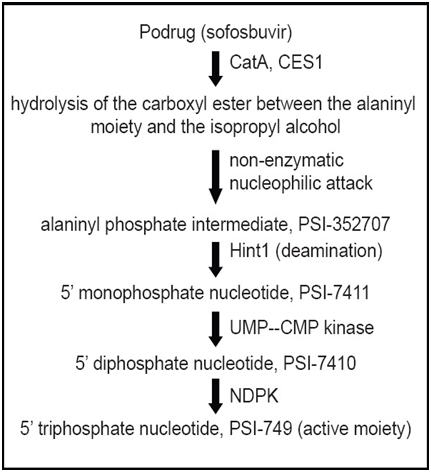
Figure 4Metabolic pathway of Sofosbuvir.8
Spectrophotometric Methods: Khedkar PM et al.16 developed UV-spectrophotometric method for the estimation of Sofosbuvir in bulk and tablet dosage form. The analytical method was validated for various parameters as per ICH guidelines. Methanol was used as solvent. Sofosbuvir exhibit absorption maxima at 260nm. The developed method obeyed Beer-Lambert’s law having line equation Y=0.023x-0.012. The result parameters are tabulated in Table 5.16
Hamd-El et al.17 developed validated UV-spectrometric method for determination of Sofosbuvir in tablet dosage form. SOF was dissolved in methanol at λmax of 260nm. The regression equations obtained by least squared was Y=0.0157x-0.039. The result parameters are tabulated in Table 6.17
Parameters |
Result |
Linearity range (µg/ml) |
5-100 |
Correlation coefficient |
0.999 |
%Recovery (%) |
98.23 |
Precision intraday (%RSD) |
1.80 |
Limit of detection (µg/ml) |
1.6 |
Limit of quantification (µg/ml) |
4.8 |
Table 6 Validation parameters reported by Hamd-El et al.17
Abdel S et al.18 reported a direct UV spectrophotometric method and validated for the quantification of Sofosbuvir. This method was performed at 260nm using methanol as solvent. %Assay was found to be 99.74±0.654. The result parameters are tabulated in Table 7.18
Parameters |
Result |
Linearity range (µg/ml) |
5-40 |
Correlation coefficient |
0.9997 |
%Recovery (%) |
100.96 |
Precision intraday (%RSD) |
100.94 |
Limit of detection (µg/ml) |
1.50 |
Limit of quantification (µg/ml) |
4.50 |
Content uniformity (%) |
98.96 |
Table 7 Validation parameters reported by Abdel S et al.18
Omprakash B et al.19 has developed and validated study of UV spectrophotometric method for determination of Sofosbuvir in bulk and pharmaceutical dosage forms. The solvent system consisted of Water & Ethanol in the ration of 60:40v/v and the wavelength to maximum absorbance at 260nm. Analytical calibration curves were linear within a concentration range from 2 to 12μg/ml and coefficient of correlation 0.997. %RSD was found to be less than 2. The result of analysis has been validated statistically.19
Chakravarthy A et al.20 developed and validated the method for estimation of Daclatasvir and Sofosbuvir in active pharmaceutical ingredient. The measurement of Daclatasvir and Sofosbuvir was done at 317nm and 261nm respectively using methanol as a solvent. The %assay of Dactlatasvir and Sofosbuvir was found to be 99.4% and 99.8%. The result parameters are tabulated in Table 8.20
Parameters |
Daclatasvir |
Sofosbuvir |
Linearity range (µg/ml) |
50-150 |
43-143 |
Correlation coefficient |
0.99 |
0.99 |
%Recovery (%) |
99.4-100.6 |
99.7-100.6 |
Repeatability (%RSD) |
0.32 |
0.17 |
Table 8 parameters reported by Chakravarthy A et al.20
Abdel S et al.21 developed validated simultaneous spectrophotometric quantification of a new antiviral combination. In the first method, the two drugs were determined simultaneously using first derivative (D1) method. It was accomplished by measuring peak heights at 275nm and 344nm, for SFV and LDI, respectively, in concentration ranges of 5‐80μg/mL and 3‐50μg/mL, for SFV and LDI, respectively. In the second one, a first derivative of ratio spectra (1DD) method was adopted to quantify SFV in concentration range of 5‐80μg/ml. It was adopted by measuring the peak amplitudes at 259nm and 280nm, using 25μg/ml LDI as a divisor. The proposed method was also used to determine LDI in concentration range of 3 ‐50μg/ml by recording the peak amplitudes at 319nm and 375nm, using 80μg/ml SFV as a divisor. The result parameters are tabulated in Table 9.21
Parameter |
SFV |
SFV |
LDI |
LDI |
Linearity range (µg/ml) |
5-80 |
5-80 |
3-50 |
3-50 |
Correlation coefficient |
0.9998 |
0.9997 |
0.9999 |
0.9999 |
%Recovery (%) |
101.24 |
98.54 |
100.57 |
99.18 |
Precision intraday (%RSD) |
98.88 |
99.22 |
98.89 |
98.29 |
Precision interday (%RSD) |
99.18 |
103.78 |
100.54 |
102.87 |
Limit of detection (µg/ml) |
2.87 |
2.49 |
0.89 |
1.00 |
Limit of quantification (µg/ml) |
4.97 |
4.90 |
2.97 |
2.89 |
Content uniformity |
101.31 |
99.67 |
99.2 |
102.21 |
Table 9 Validation parameters reported by Abdel S et al.21
Rai S et al.22 reported a UV-spectroscopy method for simultaneous estimation of Sofosbuvir and Ledipasvir in their combined tablet dosage form. Spectrophotometric methods for simultaneous estimation are developed & validated using Q-absorbance ratio method. In Q-Absorbance ratio method Sofosbuvir and Ledipasvir showed an iso-absorptive point at 241nm, the second wavelength used was 260nm, which was λmax of Sofosbuvir. The %assay of Sofosbuvir and Ledipasvir was found to be 99.74% and 100.05% respectively. The result parameters are tabulated in Table 10.22
Parameters |
Sofosbuvir |
Ledipasvir |
||
|
260 |
241 |
260 |
241 |
Linearity range (µg/ml) |
10-30 |
10-30 |
2.25-6.75 |
2.25-6.75 |
Correlation coefficient |
1.0000 |
0.9989 |
0.9995 |
0.9998 |
%Recovery (%) |
99.55-100.51 |
100.16-100.20 |
||
Repeatability (%RSD) |
0.34 |
0.65 |
0.81 |
0.97 |
Precision intraday (%RSD) |
0.69 |
0.52 |
||
Precision interday (%RSD) |
0.52 |
0.36 |
||
Limit of detection (µg/ml) |
0.139 |
0.975 |
0.143 |
0.094 |
Limit of quantification (µg/ml) |
0.412 |
2.955 |
0.433 |
0.284 |
Table 10 Validation parameters reported by Rai S et al.22
Abdelwahab NS et al.23 developed innovative spectroscopic methods for determination of newly discovered combination for Hepatitis C treatment. In this work novel spectrophotometric methods were developed for resolving the partially overlapped spectra of LED and SOF with simple data manipulation and without preliminary separation steps. In method (I), LED was directly determined using it extended spectra at 325nm where no interference from the co-formulated SOF while the absorption at the isoabsorptive point (λ=262.4nm) was used for measuring concentrations of both. By subtraction, concentration of SOF could be obtained. Method (II) is the absorbance subtraction method (AS) at which a mathematically estimated factor representing the absorbance ratio (A262.4/A325) for pure LED was used for simultaneous quantitation of LED and SOF using a unique equation computed at λiso (262.4nm). Method (III) depended on using ratio spectra and then measuring the amplitude of the constant at 325nm for LED while using ratio subtraction spectrophotometric method to quantify SOF at 262nm. Finally, method (IV) was area under the curve correction method at which the areas from 245-265 and 315-335nm and a mathematically calculated factor for pure LED were used. The result parameters are tabulated in Table 11.23
Parameters |
Method 1 |
Method 2 |
Method 3 |
Method 4 |
||||
LED |
SOF |
LED |
SOF |
LED 325nm |
SOF |
LED |
SOF |
|
Linearity range (µg/ml) |
2-25 |
2-50 |
2-25 |
2-50 |
2-25 |
2-50 |
2-25 |
2-50 |
Correlation coefficient |
0.9998 |
- |
0.9999 |
- |
0.9998 |
0.9999 |
0.9998 |
0.9999 |
%Recovery (%) |
99.94 |
99.61 |
100.85 |
99.61 |
99.87 |
99.67 |
98.62 |
99.95 |
Precision Interaday (%RSD) |
1.280 |
1.104 |
2.154 |
1.126 |
1.41 |
1.739 |
0.740 |
1.631 |
Precision interday (%RSD) |
1.739 |
1.918 |
2.169 |
1.968 |
2.483 |
1.982 |
2.127 |
1.983 |
%Assay |
102.13 |
101.66 |
101.75 |
102.25 |
100.66 |
101.06 |
101.76 |
100.46 |
Table 11 Validation parameters reported by Abdelwahab NS et al.23
Fotouh RM et al.24 developed innovative spectroscopic method for the simultaneous determination of Sofosbuvir and Ledipasvir. The zero order spectra of SBV and LPV show that LPV has a λmax at 333nm, while SBV has a λmax at 260nm. Simultaneous determination of Sofosbuvir and Ledipasvir in their binary mixtures was performed using two methods; a direct UV spectrophotometric method for determination of Ledipasvir at 333nm, and the new “wavelength-intersection ratio” method for determination of Sofosbuvir. In the wavelength-intersection ratio method, different mixtures of Sofosbuvir and Ledipasvir containing different concentration ratios were prepared; the zero crossing point of the first derivative curve in the range 285 to 295nm were determined for each mixture. An absorbance shift in the intersection was obtained with the change in the concentration ratio (Sofosbuvir/Ledipasvir).The zero order spectra were measured, the first derivative was calculated for each mixture and the intersection wavelengths were compared. %Assay was found to be 100.14% and 99.80% for Ledipasvir and Sofosbuvir, respectively. The result parameters are tabulated in Table 12.24
Parameters |
Sofosbuvir |
Ledipasvir |
Linearity range (µg/ml) |
11-110 |
3-18 |
Correlation coefficient |
0.9992 |
0.9992 |
%Recovery (%) |
99.80 |
100.14 |
Precision interaday (%RSD) |
0.57 |
1.79 |
Precision interday (%RSD) |
0.76 |
1.88 |
Limit of detection (µg/ml) |
3 |
0.5 |
Limit of quantification (µg/ml) |
11.0 |
3.0 |
Table 12 Validation Parameters reported by Fotouh RM et al.17
Eissa MS et al.25 developed simultaneous determination of Sofosbuvir and Ledipasvir using smart spectrophotometric methods manipulating ratio method. In this work, various sensitive and selective spectrophotometric methods were first introduced for the simultaneous determination of sofosbuvir and ledipasvir in their binary mixture without preliminary separation. Ledipasvir was determined simply by zero-order spectrophotometric method at its λmax=333.0nm in a linear range of 2.5-30.0μg/ml without any interference of sofosbuvir even in low or high concentrations and with mean percentage recovery of 100.05±0.632. Sofosbuvir can be quantitatively estimated by one of the following smart spectrophotometric methods based on ratio spectra developed for the resolution of the overlapped spectra of their binary mixture; ratio difference spectrophotometric method (RD) by computing the difference between the amplitudes of sofosbuvir ratio spectra at 228nm and 270nm, first derivative (DD1) of ratio spectra by measuring the sum of amplitude of trough and peak at 265nm and 277nm, respectively, ratio subtraction (RS) spectrophotometric method in which sofosbuvir can be successfully determined at its λmax=261.0nm and mean centering (MC) of ratio spectra by measuring the mean centering values at 270nm. All of the above mentioned spectrophotometric methods can estimate sofosbuvir in a linear range of 7.5-90.0μg/ml with mean percentage recoveries of 100.57±0.810, 99.92±0.759, 99.51±0.475 and 100.75±0.672, respectively. These methods were successfully applied to the analysis of their combined dosage form and bulk powder. The adopted methods were also validated as per ICH guidelines and statistically compared to an in-house HPLC method.25
Abo T et al.26 has developed spectrophotometric methods for simultaneous determination of Sofosbuvir and Ledipasvir . These methods were based on direct measurement of ledipasvir at 333nm. Concentration range of 4.0–14.0µg/mL, with a mean recovery of 100.78. Sofosbuvir was determined, without prior separation, by third-derivative values at 281 nm; derivative ratio values at 265.8nm utilizing 5.0µg/mL ledipasvir as a divisor; the ratio difference method using values at 270 and 250nm using 5.0µg/mL ledipasvir as a divisor; and the ratio subtraction method using values at 261nm. These methods were found to be linear for sofosbuvir over a concentration range of 5.0–35.0µg/mL. Statistical analysis of the results showed no significant difference between the proposed methods and the manufacturer's LC method of determination with respect to accuracy and precision. The analytical method was validated for various parameters as per ICH guidelines.26
Chromatographic methods: The high performance liquid chromatography (HPLC) method has been reported for the analysis of Sofosbuvir in pharmaceutical formulation, bulk as well as in biological fluids.
High performance liquid chromatography (HPLC): Vikas M et al.27 developed and validated new RP- HPLC method for the determination of Sofosbuvir in pure form. Separtion of SFS was successfully achieved on a Hisil C18 (4.6 x 250mm, 5μm) column by Waters or equivalent in an isocratic mode utilizing Phosphate Buffer (4.0pH): Methanol (50:50%v/v) at a flow rate of 0.8mL/min and eluate was monitored at 262nm. The volume of injection loop was found to be 10µl.The retention time was found to be 1.01min. The validation parameters are tabulated in Table 13.27
Parameters |
Result |
Linearity range (µg/ml) |
5-30 |
Correlation coefficient |
1.000 |
Precision intraday (%RSD) |
0.19 |
Table 13 Validation parameters reported by Vikas M et al.20
Jeyabaskaran M et al28 developed and validated a new RP-HPLC method for Sofosbuvir in bulk and pharmaceutical dosage form. The estimation of Sofosbuvir (SOF) done in bulk and pharmaceutical dosage forms using a Kromasil C18 (250mm×4.6mm, 5μ) column with temperature 25◦C and mobile phase comprising 0.1% Ortho phosphoric acid buffer and acetonitrile in the ratio 55:45(v/v). The flow rate was 1ml/min and detection was carried out by photodiode array detector at 260nm. The retention time was found to be 2.06min. The %assay was found to be 100.50%. The validation parameters are tabulated in Table 14.28
Parameters |
Result |
Linearity range (µg/ml) |
100-600 |
Correlation coefficient |
0.9991 |
%Recovery (%) |
99.10-101.74 |
Precision intraday (%RSD) |
0.3 |
Limit of detection (µg/ml) |
0.762 |
Limit of quantification (µg/ml) |
2.308 |
Table 14 Validation parameters reported by Jeyabaskaran M et al.21
Bhimana S et al.29 developed high performance liquid chromatographic method for the determination of Sofosbuvir in pharmaceutical dosage form. This method uses Agilent Eclipse XDB-C18 (5μm, 4.6x250mm) analytical column, a mobile phase of acetonitrile: potassium dihydrogen phosphate buffer pH 2.5 adjusted with orthophosphoric acid in ratio (55:45v/v). The instrumental settings are a flow rate of 1.0ml/min and Photon Diode Array detector wavelength at 260nm. The retention time was found to be 3.16min. The %assay was found to be 99.5%. The validation parameters are tabulated in Table 15.29
Parameters |
Result |
Linearity range (µg/ml) |
140-420 |
Correlation coefficient |
0.999 |
%Recovery (%) |
87.81-112.36 |
Precision (%RSD) |
0.043 |
Limit of detection (µg/ml) |
0.035 |
Limit of quantification (µg/ml) |
1.05 |
Table 15 Validation parameters reported by Bhimana S et al.22
Vejendla R et al.30 developed validation of Sofosbuvir by RP-HPLC method in bulk and tablet dosage form. To optimize, a column Phenomenex prodigy ODS-3V (150mm x4.6mm, 5μm), mobile phase mixture of methanol and (0.1%) tri-fluro acetic acid as buffer having pH of 3.2 in the ratio of (30:70v/v) found to be an efficient system for elution of drug with good peak shape with flow rate 1.0ml/min at UV wavelength of 260nm. The retention time was found to be 2.989min. The %assay was found to be 99.81%. The validation parameters are tabulated in Table 16.30
Parameters |
Result |
Linearity range (µg/ml) |
100-600 |
Correlation coefficient |
0.9964 |
%Recovery (%) |
99.35 |
Precision intraday (%RSD) |
0.239 |
Limit of detection (µg/ml) |
0.05 |
Limit of quantification (µg/ml) |
0.15 |
Table 16 Validation parameters reported by Ravikumar et al.23
Panchumarthy R et al.31 developed and validated a rapid RP-HPLC method for the determination of Sofosbuvir in bulk and in pharmaceutical dosage form. Agilent High Pressure Liquid Chromatography 1260 series with GI311C Quat. Pump Eclipse XDB-C18 Colum (5μm particle sizex4.6×250mm) and diode array detector G1315D was utilized in the study. The mobile phase consisting of methanol and acetonitrile in the proportion of 30:70v/v was used for the study. A flow rate of 1mL/min with an injection volume of 20μL was selected for this study. The separation was acquired at a temperature of 30◦C and eluents were observed using by photo diode array detector set at 261nm. The retention time was found to be 2.40min. The %assay was found to be 99.3%. The validation parameters are tabulated in Table 17.31
Parameters |
Result |
Linearity range (µg/ml) |
10-30 |
Correlation coefficient |
0.9998 |
%Recovery (%) |
99.3-99.9 |
Precision repeatability (%RSD) |
0.011 |
Limit of detection (µg/ml) |
0.9708 |
Limit of quantification (µg/ml) |
2.9420 |
Table 17 Validation parameters reported by Panchumarthy R et al.24
Abdel S et al.32 reported a reversed phase high‐performance liquid chromatographic (RP‐HPLC) validated for the quantification of Sofosbuvir. The RP‐HPLC method was applied on Hypersil TM ODS C18 column (150×4.6mm, 5μm) as a stationary phase. The mobile phase was methanol: acetonitrile (90:10,v/v), pumped using an isocratic mode with flow rate of 1mL/min and UV detection at 260nm. The retention time was found to be 1.99min. The validation parameters are tabulated in Table 18.32
Parameters |
Result |
Linearity range (µg/ml) |
2-60 |
Correlation coefficient |
0.9996 |
%Recovery (%) |
98.94 |
Precision intraday (%RSD) |
1.12 |
Limit of detection (µg/ml) |
0.25 |
Limit of quantification (µg/ml) |
1.7 |
Content uniformity |
102.08 |
Table 18 Validation parameters reported by Abdel S et al.25
Guguloth R et al.33 has developed and validated RP-HPLC of Sofosbuvir tablet. The method utilized RP-HPLC (Water 2695 with PDA detector) model and a column Agilent C18 4.5×100mm 3.0μm. The mobile phases were comprised with 60:40 of Methanol: Water at a flow rate of 1.0ml/min. UV detection at 235nm MTS were eluted with retention times of 2.351min. The retention time was found to be 2.351min. The Accuracy limit is the % recovery should be in the range of 99.1-99.9%. The result parameters are tabulated in Table 19.33
Parameters |
Result |
Linearity range (µg/ml) |
320-480 |
Correlation coefficient |
0.9993 |
Sysytem suitability (%RSD) |
0.31 |
Method repeatability (%RSD) |
0.75 |
%recovery (%) |
99.1-99.9 |
Table 19 Validation Parameters reported by Gugguloth R et al.26
Swathi P et al.34 has developed RP-HPLC method and validation for estimation of Sofosbuvir in pure and tablet form. The important features and novelty of the proposed method included simple sample treatment with sonicator of small amount of powder sample at ambient temperature. In High Performance Liquid Chromatography (Waters 2695 HPLC, Class) with 2487 pumps, auto injector with loop volume of 10μl (Rheodyne), programmable variable wavelength PDA detector. The mobile phase was Methanol (100%) and flow rate was 1.0min/ml. The detection wavelength was 265nm. The retention time was found to be 3.512min. The result parameters are tabulated in Table 20.34
Parameters |
Result |
Linearity Range (µg/ml) |
20-100 |
Correlation Coefficient |
0.9997 |
Precision (%RSD) |
0.13 |
System suitability (%RSD) |
0.24 |
Table 20 Validation parameter reported by Swathi P et al.28
El-Yazbi AF et al.35 developed a comparative validation of Sofosbuvir determination in pharmaceuticals by several chromatographic, electrophoretic and spectrophotophotometric method. In this work five accurate methods for the determination of sofosbuvir in tablets: reversed phase high pressure liquid chromatography (RP-HPLC), capillary zone electrophoresis (CZE), high performance thin layer chromatography (HPTLC) with densitometric detection, UV spectrophotometric and derivative spectrometry methods, were developed and validated. The HPLC was carried out using C18 Thermo stationary phase and mobile phase consisted of 0.1% formic acid-acetonitrile (60: 40 v/v) with flow rate 1mL min−1 and UV detection at 260nm. CZE was performed using 75μm×82cm fused silica capillary. Detection was carried out at 230nm with 10mM phosphate buffer pH 7.50, 30kV voltage and 25◦C temperature. NP-HPTLC was carried out using HPTLC silica F254 plates, developed with methanol–chloroform (70:30, v/v) through 19cm distance. Analysis were scanned with densitometer at 260nm. UV spectrophotometry was carried out using 260nm for direct assay and 215 and 245nm for the first derivative assay. The proposed methods proved to be rapid, simple, sensitive, selective and accurate analytical procedures, suitable for reliable determination of sofosbuvir in tablets for routine quality control.35
Rai S et al.36 developed and validated RP-HPLC for Sofosbuvir and Ledipasvir in their combined tablet dosage form. The separation was achieved by BDS Hypersil C18 column (150X4.6mm, 5μm) column, and ACN: 0.1% TFA in the proportion of 32:68 %v/v as mobile phase, at a flow rate of 1ml/min. Detection was carried out at 245nm. The retention time was found to be 2.37min and 5.49min of Sofosbuvir and ledipasvir respectively. The %assay of Sofosbuvir and Ledipasvir was found to be 99.74% and 100.05% respectively. The validation parameters are tabulated in Table 21.36
Parameters |
Sofosbuvir |
Ledipasvir |
Linearity range (µg/ml) |
100-600 |
22.5-135 |
Correlation coefficient |
1.0000 |
1.0000 |
%Recovery (%) |
99.92 |
100.45 |
Precision intraday (%RSD) |
0.55 |
0.53 |
Limit of detection (µg/ml) |
0.395 |
0.132 |
Limit of quantification (µg/ml) |
1.197 |
0.401 |
Table 21 Validation parameters reported by Rai S et al.29
Nagaraju T et al.37 has developed RP-HPLC for the simultaneous assay of Sofosbuvir and Ledipasvir in combined dosage form. The isocratic mobile phase consisted of buffer (pH -2.0), acetonitrile and methanol (30:50:20% v/v/v), flowing through the Inertsil ODS C18 column (make: 150mmx4.6mm i.d; particle size 5μm) at a constant flow rate of 1.0ml/min at ambient column temperature with a sample injection volume of 10μl. Detection of the analytes (sofosbuvir and ledipasvir) were carried out at a wavelength of 267nm. The retention time of Sofosbuvir and Ledipasvir was found to be 3.205 and 3.774min respectively. The %assay of Sofosbuvir and Ledipasvir was found to be 99.9% and 99.98% respectively. The validation parameters are tabulated in Table 22.37
Parameters |
Ledipasvir |
Sofosbuvir |
Linearity range (µg/ml) |
40-120 |
10-30 |
Correlation coefficient |
0.999 |
0.999 |
%Recovery (%) |
99.2-100.9 |
98.40-100.9 |
Precision intraday (%RSD) |
0.77 |
0.84 |
Limit of detection (µg/ml) |
0.015 |
0.012 |
Limit of quantification (µg/ml) |
0.050 |
0.042 |
Table 22 Validation Parameters reported by Nagaraju T et al.30
Raj Kumar B et al.38 developed and validated a new RP-HPLC method for the simultaneous determination of Simeprevir and Sofosbuvir in pharmaceutical dosage form. In this method using X-Terra C18 as stationary phase and a mobile phase containing a mixture of Acetonitrile: Water (75:25% v/v). Run time was selected to be 10min. The flow rate was 1.0ml/min and effluent was monitored at 253nm and column oven temperature was maintained ambient. The retention time was found to be 5.289min and 2.09min of Sofosbuvir and Simeprevir respectively. The validation parameters are tabulated in Table 23.38
Parameters |
Simeprevir |
Sofosbuvir |
Linearity range (µg/ml) |
7-35 |
18.2-91 |
Correlation coefficient |
0.999 |
0.9979 |
%Recovery (%) |
98-102 |
98-102 |
Repeatability precision (%RSD) |
0.6 |
0.2 |
Limit of detection (µg/ml) |
0.95 |
6.5 |
Limit of quantification (µg/ml) |
2.8 |
19.9 |
Table 23 Validation parameters reported by Raj Kumar B et al.31
Kranthi KK et al.39 developed and validated new analytical method for simultaneous estimation of Ledipasvir and Sofosbuvir using RP-HPLC. Chromatographic separation was achieved on a c18 column using mobile phase consisting of a mixture of Mixed Phosphate Buffer: Acetonitrile (55:45) with detection of 213nm. These studies were carried out at 250C and 6.8pH. The flow rate and injection volume was 1.0ml/min and 20µl respectively. The retention time was found to be 7.453min and 3.60min of Sofosbuvir and Ledipasvir respectively. The %assay of Sofosbuvir and Velpatasvir was found to be 101.97% and 99.56% respectively. The proposed methods were validated, accurate and precise. The validation parameters are tabulated in Table 24.39
Parameters |
Ledipasvir |
Sofosbuvir |
Linearity range (µg/ml) |
60-140 |
6-14 |
Correlation coefficient |
0.999 |
0.996 |
%Recovery (%) |
99.59% |
100.43% |
Repeatability (%RSD) |
0.92 |
1.63 |
%Assay |
99.56 |
101.97 |
Table 24 Validation parameters reported by Kranthi KK et al.32
Zaman B et al.40 has developed RP-HPLC method for simultaneous determination of Sofosbuvir and Ledipasvir in tablet dosage form and its application to in vitro dissolution studies The analysis was performed on Luna analytical column 250×4.6mm, 5µm, octyl silica packing (Si–[CH2]7–CH3) C8, using ammonium acetate buffer solution pH 7.0 and acetonitrile 35:65% v/v as mobile phase at flow rate of 0.7mL min−1 for isocratic elution. Detection of sofosbuvir and ledipasvir was performed on a UV detector at 245nm. The retention times of sofosbuvir and ledipasvir were 4.468±0.013min and 8.242±0.012min, respectively, and the total run time was 20min. The result parameters are tabulated in Table 25.40
Parameters |
Sofosbuvir |
Ledipasvir |
Correlation coefficient |
0.9999 |
0.9999 |
%Recovery |
100 |
100 |
Precision intraday (%RSD) |
2 |
2 |
Precision Interday (%RSD) |
2 |
2 |
Limit of detection (µg/ml) |
0.485 |
0.175 |
Limit of quantification (µg/ml) |
1.619 |
0.586 |
Table 25 Validation parameters reported by Zaman B et al.33
El-Shaboury S et al.41 has developed and validated spectrodensitometric method for simultaneous estimation of Sofosbuvir, Ribavirin and Saxagliptin in their pure and pharmaceutical dosage formulation. The method employed TLC plates precoated with silica gel G 60 F254 as the stationary phase. The mobile phase consisting of acetonitrile-water (80:20%, v/v) was used to give compact bands for all the studied drugs at 228nm. They were resolved with retardation factor (Rf) values of 0.71, 0.36 and 0.21 for sofosbuvir, ribavirin and saxagliptin respectively. The result parameters are tabulated in Table 26.41
Parameters |
Sofosbuvir |
Ribavirin |
Saxagliptin |
Linearity range (ng/band) |
400-10000 |
400-10000 |
400-10000 |
Correlation coefficient |
0.9993 |
0.9995 |
0.9991 |
Limit of detection (ng/band) |
124.78 |
124.31 |
128.29 |
Limit of quantifiaction (ng/band) |
378.13 |
376.71 |
388.77 |
Table 26 Validation parameters reported by El-Shaboury S et al.34
Hassouna M El-K et al.42 developed assay and dissolution methods development and validation for simultaneous determination of Sofosbuvir and Ledipasvir by RP-HPLC method in tablet dosage forms. RP-HPLC method was performed on the Eclipse XDB C18 column (250mmX4.6mm, 5μm particle size, using buffer solution of pH 3.0 containing 0.02M potassium dihydrogen phosphate and 5.7mM hexane sulfonate : acetonitrile (50:50 v/v) as the mobile phase at a flow rate of 1.5ml/min, injection volume 10μL and UV detection at 254 nm. The retention time was found to be 2.429min and 4.259min of Sofosbuvir and Ledipasvir respectively. The %assay of Sofosbuvir and Ledipasvir was found to be 99.33% and 99.51% respectively. This method is validated according to BP, USP and ICH requirements for new methods, which include accuracy, precision, selectivity, robustness, ruggedness, LOD, LOQ, linearity and range. The forced degradation studies as acidity, alkalinity, oxidation, heat, thermal, humidity and photo degradation were performed according to ICH guidelines. The validation parameters are tabulated in Table 27.42
Parameters |
Sofosbuvir |
Ledipasvir |
Linearity range (µg/ml) |
40-500 |
30-300 |
Correlation coefficient |
0.999 |
0.9996 |
%Recovery |
111.12 |
99.51 |
Intraday (%RSD) |
1.93 |
1.95 |
Interday (%RSD) |
0.685 |
0.649 |
Limit of detection (µg/ml) |
4.14 |
3.64 |
Limit of quantification (µg/ml) |
12.54 |
11.03 |
Table 27 Validation parameters reported by Hassouna M El-K et al.35
Madhavi S et al.43 developed and validated a simple methodology to quantify the most used drug Sofosbuvir for the treatment of hepatitis C virus (HCV) infection, in human plasma by using Atazanavir as an Internal Standard (IS) for preclinical studies and validate as per USFDA guidelines. Sofosbuvir was isolated from plasma samples by liquid-liquid extraction method using acetonitrile; good chromatographic separation was achieved on Kromasil Column (250mm×4.6mm, 5μm). The mobile phase consisted of 0.1% orthophosphoric acid (OPA) buffer pH2 and acetonitrile in the ratio of (68:32, v/v), respectively. The analysis time was 7min at a flow rate 1ml/min. The photodiode array detector (PDA) detection was carried out at 228nm. The retention time was found to be 4.7min. The validation parameters are tabulated in Table 28.43
Parameters |
Result |
Linearity range (µg/ml) |
0.050-2.0 |
Correlation coefficient |
0.999 |
%Recovery (%) |
84.14 |
Precision intraday (%RSD) |
1.7 |
Limit of quantification (µg/ml) |
0.050 |
Table 28 Validation parameters reported by Madhavi et al.43
RP-HPLC-DAD: Farid NF et al.44 has developed chromatographic analysis of Ledipasvir and Sofosbuvir in human plasma. A reversed phase high performance liquid chromatography - diode array detector (RP-HPLC/DAD) method was developed and validated. In the developed method, separation was performed on Zorbax® Eclipse C18 column using a gradient mixture of acetonitrile–water as a mobile phase and scanning was performed at 260nm (for SOF) and 330nm (for LED). The two drugs were completely separated from each other and from plasma, where plasma peak appeared at 2.76±0.05 min, SOF at 4.25±0.05, and LED at 7.35±0.05. The developed method showed high sensitivity, the drugs showed linearity in the range of 1–45µg/ml for both pure form and spiked human plasma. Three freeze–thaw cycles were performed separately at two different temperatures, −8 and −20°C. Validation parameters such as accuracy, precision, robustness, and ruggedness were tested in compliance with USP recommendation.44
HPLC-MS-MS: Rower JE et al.45 has developed serum and cellular Ribavirin pharmacokinetic concentration effect analysis in HCV patients receiving Sofosbuvir plus Ribavirin. Individuals infected with HCV genotype 1 (GT1) received 400mg of sofosbuvir and either low-dose or weight-based ribavirin as part of the NIAID SPARE trial. Ribavirin serum levels were quantified using validated HPLC-MS/MS method linear between 0.05 to 10mg.ml. Whole blood was drawn into PAXgene RNA isolation tubes, stored at -80◦C and then diluted 1:50 with 1mL of 70% methanol solution prior to extraction. These samples were treated as isolated RBC samples during data analysis, as a strong correlation was established to concentration results from purified RBC samples. The regression Coefficient was found to be 0.9984. Samples were separated into RBV-MP and RBV-TP fractions using a Waters QMA strong anion-exchange solid-phase extraction (SPE) cartridge, dephosphorylated and then prepared for a validated HPLC-MS/MS analysis using a Varian BondElut Phenylboronic Acid SPE cartridge. The assay was linear between 0.5 and 200 pmol/sample and reported concentrations are normalized to a per million cell count (pmol/ 106 cells).45
Nebsen M et al.46 has developed stability indicating method and LC-MS-MS characterization of forced degradation products of Sofosbuvir. A rapid specific RP-HPLC method was developed. Sobosbuvir was subjected to hydrolysis (acidic, alkaline and neutral), oxidation, photolysis and thermal stress. The drug showed degradation under oxidative, photolysis, acid and base hydrolysis stress conditions. Chromatographic separation of the drug from its degradation products was performed on Inertsil ODS-3 C18 (250mm×4.6mm i.d., 5µm) column using a green mobile phase of methanol:water 70:30 (v/v). The degradation products were characterized by LC–MS-MS and the fragmentation pathways were proposed. The developed method was validated as per ICH guidelines.46
Ultra performance liquid chromatography (UPLC): Pottabathini V et al.47 reported new stability indicating reverse phase chromatographic method for analysis of Sofosbuvir. The developed UPLC method was superior in technology to conventional RP-HPLC with respect to resolution, speed, solvent consumption and analysis cost. Sofosbuvir was subjected to the thermal, hydrolytic, oxidative, and photolytic degradation, according to ICH guidelines. Photo diode array detector was selected to develop the stability indicating method and isolation of degradation products. The wavelength 260nm was selected and sample solutions prepared in clear volumetric flasks were stable up to 30 days in temperature from 2˚C to 8˚C. The X Bridge C18 (100. 4.6) mm 2.5μ was used for good sepration. Combination of acetonitrile and 0.1% Formic acid buffer achieved good separation with flow rate was 0.2ml/min. The retention time was found to be 5.14min. The %assay was found to be 99.84%.The validation parameters are tabulated in the Table 29.47
Parameters |
Result |
Linearity range (µg/ml) |
5-25 |
Correlation coefficient |
0.999 |
%Recovery (%) |
99.62 |
Precision intraday (%RSD) |
0.253 |
Limit of detection (µg/ml) |
0.27 |
Limit of quantification (µg/ml) |
0.83 |
Table 29 Validation parameters reported by Pottabathini V et al.47
UPLC-MS-MS: Bhatt D et al.48 has developed UPLC-MS/MS method and validated for the estimation of Sofosbuvir from human plasma. Samples prepared by employing liquid-liquid extraction (LLE) using 2.5ml of ethyl acetate. Chromatographic separation was achieved on Gemini 5μ C18, 50x4.6mm column using a mixture of 0.1% (v/v) formic acid in water to methanol at a ratio of 30:70 v/v as the mobile phase. The flow rate was 0.50ml/min. The LC eluent was split, and approximately 0.1ml/min was introduced into Tandem mass spectrometer using turbo Ion Spray interface at 325°C. Quantitation was performed by transitions of 428.35/279.26 (m/z) for sofosbuvir and 431.38/282.37 (m/z) for sofosbuvir-d3. Chromatographic separation was achieved within 2min. The %assay was found to be 94.22%. The result parameters are tabulated in Table 30.48
Parameters |
Result |
|
Linearity range (µg/ml) |
4.063-8000.01 |
|
Correlation coefficient |
0.9985 |
|
Ruggedness (%) |
0.35-3.09 |
|
%Stability (short term) |
97.25 |
|
%Recovery |
LQC |
75.47 |
MQC |
74.37 |
|
HQC |
76.26 |
|
Table 30 Amplitude in mill volts of the Lead-1 of electrocardiography in sheep
*Significant (P≤0.05); NSNot significant (P>0.05)
Zhenzhen P et al.49 developed a method for the simultaneous determination of Ledipasvir, Sofosbuvir and its metabolite in rat plasma by UPLC–MS/MS and its application to a pharmacokinetic study. The analytes and the internal standard (Diazepam) were separated on an Acquity UPLC BEH C18 chromatography column using gradient elution with a mobile phase of acetonitrile and 0.1% formic acid in water at a flow rate of 0.4mL/min. The detection was performed on a triple quadrupole tandem mass spectrometer by multiple reaction monitoring(MRM) mode to monitor the precursor-toproduct ion transitions of m/z 889.8→130.1 for Ledipasvir, m/z 530.3→243.1 for Sofosbuvir, m/z 261.5→113.1 for GS-331007 and m/z 285.2→193.1 for Diazepam(IS) using a positive electrospray ionization interface. Total time for each chromatography was 3.0min.49
Rezk RM et al.50 has developed novel and sensitive UPLC-MS/MS method for quantification of Sofosbuvir inhuman plasma using eplerenone as an internal standard. The Xevo TQD LC–MS/MS was operated under the multiple-reaction monitoring mode using electrospray ionization. Extraction with tert-butyl methyl ether was used in sample preparation. The prepared samples were chromatographed on Acquity UPLC BEH C18 (50×2.1mm, 1.7μm) column by pumping 0.1% formic acid and acetonitrile in an isocratic mode at a flow rate of 0.35mL/min. The standard curves were found to be linear in the range of 0.25–3500ng/mL for SF. The intra- and inter-day precision and accuracy results were within the acceptable limits. A very short run time of 1 min made it possible to analyze more than 500 human plasma samples per day. A very low quantification limit of SF allowed the applicability of the developed method for determination of SF in a bioequivalence study in human volunteers. This method was validated as per USFDA guidelines.50
UPLC-ESI-MS-MS: Rezk MR et al.51 has developed a sensitive UPLC-ESI-MS/MS method for quantification of Sofosbuvir and its metabolites, GS-331007, in human plasma using Famotidin as an internal standard. The Xevo TQD LC-MS/MS was operated under the multiple-reaction monitoring mode using electrospray ionization. Extraction with ethyl acetate was used in sample preparation. The prepared samples were chromatographed on Acquity UPLC HSS C18 (50mm×2.1mm, 1.8μm) column by pumping 0.1% formic acid and acetonitrile (50:50, v/v) in an isocratic mode at a flow rate of 0.3ml/min. The standard curves were found to be linear in the range of 10–2500 ng/ml for both SF and its metabolite. The intra-day and inter-day precision and accuracy results were within the acceptable limits. A very short run time of 1.2min made it possible to analyze more than 300 human plasma samples per day. The developed assay method was successfully applied to a bioequivalence study in human volunteers.51
Ultra high performance liquid chromatography (UHPLC): Shaik JS et al.52 developed validated ultra high performance liquid chromatography method of Sofosbuvir in its bulk and formulation form. The chromatographic separation was achieved on a Waters BEH C18 column (2.1×100mm, 1.7μm) in an isocratic elution mode with flow rate 0.4mL/min, the mobile phase of Acetonitrile and Water (30:70) in 0.1% formic acid (pH ~2-3). The retention time was found to be 2.308min. The validation parameters are tabulated in Table 31.52
Parameters |
Result |
Linearity range (µg/ml) |
20-120 |
Correlation coefficient |
0.999 |
%Recovery (%) |
99.65 |
Precision intraday (%RSD) |
0.108 |
Limit of detection (µg/ml) |
0.03 |
Limit of quantification (µg/ml) |
0.063 |
Table 31 Validation parameters reported by Shaik JS et al.52
UHPLC-MS-MS: Ariaudo A et al.53 has developed and validate a simple, fast and cheap, but still reliable UHPLC–MS/MS method for the quantification of these drugs, feasible for a clinical routine use. Solid phase extraction was performed using HLB C18 96-well plates. Chromatographic separation was performed on a BEH C18 1.7μm, 2.1mm×50mm column, settled at 50°C, with a gradient run of two mobile phases: ammonium acetate 5 mM (pH 9.5) and acetonitrile, with a flow rate of 0.4mL/min for 5min. Tandem-mass detection was carried out in positive electrospray ionization mode. Both inter and intraday imprecision and inaccuracy were below 15%, as required by FDA guidelines, while both recoveries and matrix effects resulted within the acceptance criteria. The method was tested on 80 patients samples with good performance. This method was simple, robust and precise.53
RP-UHPLC-DAD-MS: Contreras Md M et al.54 developed RP-UHPLC-DAD-MS for the qualitative and quantitative analysis of Sofosbuvir in film coated tablets and profiling degradants. This new method was based on reversed phase (RP)-ultra-high performance liquid chromatography (UHPLC) coupled to diode array detection (DAD) and mass spectrometry (MS) was developed to facilitate the qualitative and quantitative analysis of Sofosbuvir in film coated tablets. A wavelength of 260nm was selected. Multistep linear gradient was applied by using water: 0.2% formic acid as mobile phase. Separation was carried out with a Zorbax Eclipse XDB-C18 column (4.6mm×50mm, 1.8μm) (Agilent) at 24°C (column temperature). The injection volume was 2μL. The retention time was found to be 2.429min. The %assay was found to be 93%. The validation parameters are tabulated in Table 32. In this method the use of high-resolution MS enabled us to ensure the specificity, check impurities and better sensitivity.54
Parameters |
RP-UHPLC-DAD |
RP-UHPLC-MS |
Linearity range (µg/ml) |
4-250 |
0.003-4 |
Correlation coefficient |
0.999 |
0.9995 |
%Recovery (%) |
101 |
- |
Precision intraday (%RSD) |
0.4 |
7.0 |
Limit of detection (µg/ml) |
0.07 |
0.0004 |
Limit of quantification (µg/ml) |
0.36 |
0.002 |
Table 32 Validation parameters reported by Contreras Md M et al.55
High performance thin layer chromatography (HPTLC): Abdelwahab N et al.55 has developed simultaneous determination of Sofosbuvir, Paracetamol and Methionine in rat plasma using thin layer chromatography. Naphazoline HCl was used as internal standard. Complete separation between the studied components peaks and plasma peak was obtained where Rf value of MET=0.18, NAP=0.39, PAe R=0.59 and SOF=0.82. The linearity of the method was assessed over the concentrations range 160-3000ng mL-1 for both SOF and PAR and 300-3000ng mL-1 for MET. Moreover, the accuracy, intra-and inter-day precision of the quality control samples at low, medium and high concentration levels exhibited relative standard deviations (RSD)<10%. Freezing-thawing stability was also tested; additionally pharmacokinetic and pharmacodynamics co-relation of the studied drugs in animal model has been done. The developed method can be easily used during accurate monitoring of the studied drugs.48
El-Shaboury S et al.56 has developed and validated spectrodensitometric method for simultaneous estimation of Sofosbuvir, Ribavirin and Saxagliptin in their pure and pharmaceutical dosage formulation. The method employed TLC plates precoated with silica gel G 60 F254 as the stationary phase. The mobile phase consisting of acetonitrile-water (80:20%, v/v) was used to give compact bands for all the studied drugs at 228nm. They were resolved with retardation factor (Rf) values of 0.71, 0.36 and 0.21 for sofosbuvir, ribavirin and saxagliptin respectively. The result parameters are tabulated in Table 33.55
Parameters |
Sofosbuvir |
Ribavirin |
Saxagliptin |
Linearity range (ng/band) |
400-10000 |
400-10000 |
400-10000 |
Correlation coefficient |
0.9993 |
0.9995 |
0.9991 |
Limit of detection (ng/band) |
124.78 |
124.31 |
128.29 |
Limit of quantifiaction (ng/band) |
378.13 |
376.71 |
388.77 |
Table 33 Validation parameters reported by El-Shaboury S et al.56
Gas chromatography: Alzweiri M et al.57 has developed GC-MS response between analytes and denaterated analogs. Standard addition method on dimethyl azelate (DMA) and d6- dimethyl azelate (d6-DMA) was adopted to examine possible reasons for the problem. Cross contribution of mass responses, intermolecular deuterium-hydrogen exchange during chromatographic separation, and deviation in mass ionization response of C-H against C-D bonds were studied as possible reasons for this discrepancy. GC-MS analysis revealed that neither cross contribution of ions nor H2/H exchange were possible reasons behind the difference in responses between DMA and d6-DMA relying on linearity and trans-esterification studies respectively. On the other hand, a study of carbon nucleus relaxation conducted by C13-NMR depicted that energy dissipation through C-D bond is faster than that through the C-H bond; relaxation rate of carbonyl carbon in d6-DMA and DMA were 9 and 3 sec-1 respectively. Accordingly, the energy transfer through the carbon skeleton of analytes and its mass ionization degree are more efficient than those in their DA counterparts. Conclusively, GC-MS analysis of analyte, relying on the assumption of equal response with its DA, generates overestimated analytical results of analytes.56
Electrophoresis: Abdulkareem A et al.58 has developed Capillary zone electrophoresis approach for simultaneous separation and determination of Sofosbuvir and Ledipasvir in tablet. Under optimum electrophoretic conditions fused silica capillary of 57 and 50μm i.d. total length and effective length, respectively was applied for the separation of the selected drugs using 20mmol L-1 acetate buffer pH=4 as ground electrolyte and the running potential 25kV with hydrodynamic injection 5s and 70nm bar pressure. The temperature was adjusted at 25˚C and the diode array detection was carried out at 260nm. RP-HPLC and determination of SOF and LDV in tablets. The chromatographic separation was carried out under optimum conditions Eclipse XDB C18 column, mobile phase 0.02mol L-1 potassium dihydrogen phosphate of pH=3 and 5.7mmol L-1 hexane sulfonate:acetonitrile (50:50 v/v), flow rate 1.5mL min-1 with injection volume 10μL and UV detection at 254nm. The analytical parameters are tabulated in Table 34.57
Parameters |
Sofosbuvir |
Ledipasvir |
Linearity range (µg/ml) |
5-600 |
20-400 |
Correlation coefficient |
0.9995 |
0.9997 |
%Recovery (%) |
99.9 |
98.6 |
Precision intraday (%RSD) |
0.13 |
0.19 |
Limit of detection (µg/ml) |
1.5 |
6.0 |
Limit of quantification (µg/ml) |
5 |
20 |
Table 34 Validated parameters reported by Abdulkareem A et al.58
In pharmaceutical formulations and biological matters, several methods has been described for the estimation of Sofosbuvir. It can be concluded that RP-HPLC, UPLC with different detectors, UV spectrophotometry, HPTLC and electrophoresis are the most simple and easy methods for Sofosbuvir estimation in pharmaceutical formulations while HPLC-UV and LC-MS/MS, LC-ESI-MS/MS can be widely used for Sofosbuvir estimation in biological fluids like plasma, urine and serum. Thus, the current review helps researchers to widen their ideas on different improved aspects for further studies on the evaluation of the drug.
We would like to thank the management and the staff of Faculty of Pharmacy, Pioneer pharmacy Degree College for encouraging and guiding us to publish this review article. We are also grateful to the university officials for providing us with open access to various paid journals.
The author declares that there is no conflict of interest.

©2018 Reema, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.