Journal of
eISSN: 2473-0831


Research Article Volume 11 Issue 1
Department of Biochemistry, University of Port Harcourt, Nigeria
Correspondence: Kelechi N Obi, Department of Biochemistry, faculty of sciences, University of Port Harcourt, Rivers State, Nigeria
Received: January 31, 2022 | Published: February 10, 2022
Citation: Obi KN, Onyeike EN, Anacletus FC. Ficus sur methanol leaf extract ameliorated doxorubicin-induced cardio-renal damage in Wistar albino rats. J Anal Pharm Res. 2022;11(1):9-15. DOI: 10.15406/japlr.2022.11.00395
Doxorubicin is an effective and still-used antineoplastic agent. However, the clinical use of doxorubicin is marred by life-threatening cardiotoxicity and nephrotoxicity. Free radicals have been implicated in the toxicity of doxorubicin. The aim of this study was to determine the phytochemical contents and protective effects of methanol extract of Ficus sur (MEFS) in doxorubicin-induced oxidative damage in Wistar albino rats, using biomarkers of cardiac, renal functions and oxidative stress as indicators. Phytochemical analyses revealed that MEFS is rich in tannins, alkaloid, saponins, flavonoids, but low in steroids. Acute toxicity studies show that MEFS was non-toxic up to 5000mg/kg b.w of Wistar rats. Administration of doxorubicin led to a significant (P< 0.05) increase in Creatine kinase (CK-MB), troponin, creatinine, urea and MDA concentrations. Sodium and potassium concentrations were not significantly (P>0.05) affected. Cardiac and renal superoxide dismutase (SOD), catalase and glutathione (GSH) were also significantly reduced. Results also show that pre-administration of MEFS was cardio- and nephroprotective in doxorubicin oxidative damage as evidenced by significant (P<0.05) reduction of elevated troponin, CK-MB, creatinine and urea concentrations. Concentrations of renal and cardiac SOD, catalase and GSH were also significantly (p<0.05) increased by MEFS compared to doxorubicin-treated group. Cardiac and renal MDA concentrations were significantly reduced. It may be concluded that protective effects of MEFS on doxorubicin toxicity may be due to the antioxidant properties of MEFS.
Keywords: Ficus sur, antioxidant, doxorubicin, flavonoids, nephroprotective, cardioprotective
Doxorubicin (red devil) has been so effective in the treatment of haematological malignancies and soft tissue sarcomas.1 Aside nausea, vomiting, and bone marrow suppression associated with chemotherapeutic agents, doxorubicin clinical usefulness is limited due to cumulative cardiotoxicity.2 Although the mechanisms of toxicity of doxorubicin remained obscure for years, free radicals have now been implicated in congestive heart failure and impaired renal function following doxorubicin administration.3,4 Other toxic mechanisms include mitochondrial disruption and apoptosis5 and impairment of autonomic regulation of heart function.6 The selective toxicity of doxorubicin against cardiac cells is due to poor anti-oxidant defence in the cardiac tissuee.7 The toxicity of doxorubicin stems from the redox cycling capability of the quinone moiety of the drug, and by formation of doxorubicin-ferric ion complex.6 The free radicals generated from the redox cycling action cause DNA damage, lipid peroxidation and mitochondrial dysfunction leading to impairment of cardiac function.6
The only FDA approved drug for doxorubicin toxicity is dexrazoxane, which is a potent iron chelating agent. However, administration of dexrazoxane as a cardioprotective agent is, unfortunately, associated with severe neutropenia. Hence, there is need for more effective iron chelators and radical scavengers, given the fact that doxorubicin is an effective and still-used antineoplastic agent, Phenolic-rich plant extracts and flavonoid derived from them are known to modify the effects of redox cycling agents via radical scavenging, metal chelation and modulation of antioxidant enzymes in vivo.7,8 Considerable progress has been made on evaluating natural products for their protective effects on doxorubicin-induced toxicity. However, more plants and other natural products need to be evaluated in this regard.
Ficus sur (moraceae) is commonly known as fig tree. It is found mostly in terrestrial zones. It is evergreen with thick broad leaves. Ficus sur produces fruits throughout the year.9 The medicinal properties of Ficus sur are responsible for its wider applicability in treatment of wide range of diseases. A wide range of secondary metabolites including tannins, terpenoids, flavonoids, alkaloids, steroids and others have been found in Ficus sur.10 Those secondary metabolites account for the reported pharmacological properties of Ficus sur, some of which include antimicrobial,11 immunomodulatory12 and anti-leprosy.13
Plant material
The leaves of Ficus sur were collected from Abia state, Nigeria. The plant was identified at the Herbarium of University of Port Harcourt and was assigned the voucher number UPH/P/199.
Experimental rats
The experimental animals were Wistar albino rats of 8-10 weeks old purchased from the Animal House of Biochemistry Department, University of Port Harcourt.
Qualitative and quantitative phytochemical analyses
Qualitative phytochemical analyses were done by the method of Trease and Evans14 and Harborne.15
Oral acute toxicity tests
Acute toxicity test was done by the method of Lorke.16 Male Wistar albino rats were used for the study. Dose levels of 10, 100, 1000 mg/kg b.w of the methanol extracts of Ficus sur (MEFS) were used in the first stage. Within 24hr, the rats were monitored for signs of toxicity and the number of deaths was recorded. In the second phase, dose levels of 1200mg/kg b.w, 1600m/kbw, 2900mg/kg b.w and 5000m/kg b.w of the extract were used. They were observed for 24hrs for signs of toxicity and possible death. LD50 was calculated as follows: LD50 = where a is the highest dose at which no death occurred; b is the lowest dose at which death occurred.
The rats in all the groups were supplied with rat feed (pellet diet) and water ad libitum. Group 1 received 10ml/kg b.w of normal saline for 15 days. Group 2 received 10ml/kg b.w of normal saline, and on the 12th day, was given 20mg/kg b.w of Doxorubicin. Groups 3-5 received different concentrations of extracts, as indicated, for 12 days. One hour after the last extracts were given on the 12th day, 20mg/kg b.w of doxorubicin was administered intraperitoneally. The extracts were given as usual to groups 3-8 for the next 48hr. On the 15th day, the rats were sacrificed under chloroform anaesthesia. After the sacrifice, blood samples were collected in lithium heparin sample bottles for analysis. Heart and kidneys were harvested and rinsed with ice cold normal saline for post mitochondrial supernatant preparation. Organs for histopathological studies were fixed immediately with 10% formalin.
Post-mitochondrial supernatant preparation
Immediately after harvest, hearts and kidneys of the Wistar rats were rinsed and perfused with ice cold saline (0.85% w/v) and then homogenized with ice cold phosphate buffer (0.1M and pH 7.4) containing 1.17% KCl, using laboratory mortar and pestle. The homogenate was centrifuged at 10,500g for 20min to obtain supernatant devoid of mitochondria.17
Assay of catalase
Catalase activity was measured by the method of Aebi18 with slight modifications. Procedure: Two thousand microlitres of 50mM phosphate buffer (pH 7.4) was added in test tubes. This was followed by the addition of 950ul of 0.019 M hydrogen peroxide and 50ul of the tissue samples. Decrease in absorbance was continually followed at 240 nm for 2min at 30sec interval using a uv-visible spectrophotometer. Concentration of the remaining Hydrogen peroxide was estimated using the extinction coefficient of 43.59L/molcm. One unit of catalase activity is the amount of enzyme that converted 1µmol H2O2 to water and oxygen per minute.
Determination of reduced glutathione (GSH)
Reduced glutathione was determined by the method of Jollow et al.19 This is based on the reaction between 5, 5’-dithio-2-nitrobenzoic acid and sulfhydryl compounds (e.g glutathione) to generate a stable yellow compound which absorbs maximally at 412nm.
Procedure: One millilitres of 10% sulphosalicylic acid was added to 1ml the tissue samples and kept for 1hr to precipitate the proteins. This was followed by centrifugation at 1200g to remove the precipitates. Four hundred microlitre of the supernatant was added to test tubes, followed by sequential addition of 2.6ml of 0.1M phosphate buffer (PH 7.4) and 0.2ml of 100mM DTNB. The absorbance of the yellow coloured solution was determined at 412nm. The concentration of reduced glutathione in the homogenate was determined by interpolation from calibration curve established by different concentrations (10-50µM) of glutathione taken through the same procedure as the tissue samples. The results were expressed in µmol/L.
Assay of superoxide dismutase
The activity of superoxide dismutase in the samples was determined by the method of Misra and Fridovich.20 This method is based on the oxidation of adrenaline to chromogenic adenochrome by superoxide anion under basic condition. The adenochrome has an absorption maximum of 480nm.
Procedure: Two thousand five hundred microlitres (2.5 ml) of 0.05 M carbonate buffer (pH 10.2) was put in a course cuvette, followed by 100µl of the homogenate to equilibrate in the spectrophotometer. Three hundred microlitre of 0.3mM of adrenaline in (0.5% acetic acid) was added to the mixture and inverted for proper mixing. The reaction was followed continuously at 480nm for 2.5 minutes at 30sec interval. The reference cuvette contained 2.5ml of distilled water in place of the tissue samples. One unit of SOD activity was defined as the amount of SOD necessary to cause 50% inhibition of the oxidation of epinephrine to adrenochrome.
Calculation:
Increase in Absorbance per min =(A3−A0)/2.5
A0 = absorbance after 30 seconds
A3 = absorbance after 150 seconds
% Inhibition=
Determination of malondialdehyde (MDA)
Lipid peroxidation was done by the method of Esterbauer and Cheeseman21 with slight modification. This is based on the principle that MDA produced from peroxidation of unsaturated fatty acid forms pinkish pigment upon reaction with thiobarbituric acid (TBA).
Procedure: One (1) ml of tissue sample was mixed with 1 ml of 10% trichloroacetate to precipitate the proteins. Then Iml of 0.67% TBA was added. The test tubes were placed in a preheated water bath and boiled for 15mins. After cooling, the precipitates were pelleted by centrifugation. Absorbance of the pinkish chromogen was read at 532nm and the concentration of MDA calculated using the molar extinction coefficient of 153,000.
Renal and cardiac tests
Urea, creatinine, electrolytes and protein were determined according to the manufacture’s instruction enclosed in the Randox kits; while CK-MB and troponin concentrations were determined by rat-specific ELISA kits. The determinations were done using an Automated Analyzer.
Data analysis
Data were expressed as means ± standard deviation of quadruplicate determinations, and then analysed by IBM SPSS version 23 (SPSS inc. Chicago, USA). Student’s t test and One-way analyses of variance (ANOVA) and Duncan’s multiple range test were used to determine the differences among the means. Bar charts were plotted using Graphpad® Prism version 8.
Qualitative analysis
The phytochemical constituents of Ficus sur are shown in Table 1. The major phytochemical constituents are: phenolics (flavonoids, flavonols), alkaloids and saponins. These phytochemicals are present in relatively high amounts.
|
Constituents |
Ficus sur |
|
Proteins |
+ |
|
Reducing sugars |
++ |
|
Flavonoids |
+++ |
|
Alkaloid |
+ |
|
Saponins |
+++ |
|
Glycoside |
+++ |
|
Cyanogenic glycosides |
- |
|
Tannins |
++ |
|
Oil |
+ |
Table 1 Qualitative phytochemical constituents of Ficus sur
+= trace; ++ = moderate; +++ =high; - = not detected
Quantitative phytochemical constituents of MEFS
The quantitative phytochemical constituents of MEFS are presented in Table 2 below. Results show that the plant has high alkaloid, terpenoids, tannin, saponin content. moderate cardiac glycosides and low steroid contents.
|
Constitents |
Quantity (mg/g) |
|
Alkaloids |
4.19±0.21 |
|
Saponins |
5.10 ±0.64 |
|
Tannins |
5.17±0.48 |
|
Glycosides |
1.03±0.10 |
|
Terpenoids |
4.46± 0.90 |
|
Steroids |
0.28 ±0.08 |
Table 2 Quantitative phytochemical constituents of Ficus sur
Values represent Mean± standard deviation of triplicate determinations
Oral acute toxicity studies
The results of acute toxicity studies of methanol extracts of Ficus sur are presented in Table 3. Results show that within 24 hrs of administration of the extracts in phase 1, no sign of toxicity was actually observed. No death was also recorded. In phase 2 of the study, no death was recorded for MEFS.
|
Dose (mg/kg b.w) |
Survival rate (phase 1) |
|
10 |
0/3 |
|
100 |
0/3 |
|
1000 |
0/3 |
|
Survival rate (Phase 2) |
|
|
1200 |
0/2 |
|
1600 |
0/2 |
|
2900 |
0/2 |
|
5000 |
0/2 |
Table 3 Acute toxicity studies of MEFS
0/2 = no death out of two rats; 0/3= no death out of three rats
Effects of MEFS on cardiac toxicity markers in doxorubicin- induced toxicity in wistar albino rats
Table 4 shows that administration of doxorubicin caused a significant (P<0.05) increase in CK-MB and troponin concentration compared to the normal group. Pre-administration of MEFS significantly and dose-dependently reduced the CK-MB concentration compared to doxorubicin group. Pre-administration of 500mg/kg b.w of MEFS significantly (P<0.05) reduced troponin level when compared to doxorubicin group. However, 250mg/kg b.w MEFS and 750mg/kg b.w of MEFS reduced troponin level not significantly different from both normal and doxorubicin groups.
Values are Means ± standard deviation of quadruplicate determinations. Values in the same column bearing the same superscript letters are not significantly different at 5% Level.
|
Rat groups |
CK-MB (IU/L) |
Troponin (ng/ml) |
|
Normal |
70.40±6.09c |
0.58±0.12b |
|
Doxorubicin (Dox) |
162.23±7.83a |
1.03±0.09a |
|
Dox+250mg/kgb.w MEFS |
110.93±8.89b |
0.795±0.9ab |
|
Dox+500mg/kgb.w MEFS |
83.75±3.47bc |
0.67±0.44b |
|
Dox+750mg/kg b.w MEFS |
88.95±6.43bc |
0.89±0.1ab |
Table 4 Effect of methanol extracts of Ficus sur on cardiac toxicity markers in doxorubicin-induced oxidative damage in Wistar albino rats
Effects of MEFS on renal toxic markers in doxorubicin- induced toxicity in wistar albino rats
The effects of doxorubicin administration on renal toxic parameters are presented on Table 5. Results show that doxorubicin administration caused a significant increase (P<0.05) in creatinine and urea concentration compared to values in normal rat groups. Pre-administration 500mg.kg b.w of and MEFS reduced the concentration of creatinine to the level not significantly different from values in normal rat groups. However, 250mg/kg b.w and 750mg/kg b.w of MEFS reduced the concentration of creatinine to the level not significantly different from both control and doxorubicin rat groups. In case of elevated urea concentration, all the extract doses of MEFS, caused a significant reduction (P<0.05) in urea concentration compared to doxorubicin-treated rat group. Values are Means± standard deviation of quadruplicate determinations.
|
Rat groups |
Creatinine (µmol/L) |
Urea (mmol/L) |
K+ (mmol/L) |
Na+ (mmol/L) |
|
Normal |
117.28±4.33bc |
4.01±0.31d |
4.49±0.30a |
140.70±7.96a |
|
Doxorubicin |
130.28±4.48a |
11.21±2.07a |
4.75±0.19a |
138.98±7.32a |
|
Dox+250mg/kg b.w MEFS |
125.48±2.60ab |
7.48±1.52b |
5.0±0.15a |
137.85±5.67a |
|
Dox+500mg MEFS |
104.58±6.85c |
4.79±0.98d |
4.68±0.38a |
135.93±5.06a |
|
Dox +750mg/kg b.w MEFS |
122.92±4.75ab |
6.68±1.09bc |
4.48±0.30a |
144.65±7.18a |
Table 5 Effect of methanol extracts of ficus sur on renal toxicity markers in doxorubicin-induced oxidative damage in Wistar albino rats
Values in the same column bearing the same superscript letters are not significantly different at 5% Level
Effects of MEFS on cardiac and renal antioxidant enzymes in doxorubicin oxidative stress
The effects of MEFS on cardiac superoxide dismutase (SOD) and Catalase are shown in Figures 1 and 2 respectively. Compared to the control group, Doxorubicin administration significantly reduced (P< 0.05) the activities of SOD and catalase in the cardiac tissue and renal tissues. Pre-treatment of rats with MEFS significantly enhanced the activities of the cardiac and renal SOD. In the cardiac tissues, 750mg/kg b.w MEFS was less effective in increasing SOD concentration while in the renal tissues, 250mg/kg b.w MEFS was less effective in increasing SOD concentration. Pre-administration of MEFS dose-dependently increased the activities of cardiac catalase; however, in the renal tissues, catalase activities increased but most effectively at 500mg/kg b.w MEFS.
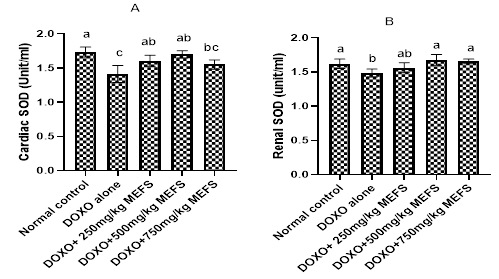
Figure 1 The effects of MEFS on cardiac and renal SOD activities in doxorubicin- induced oxidative stress in Wistar albino rats.
Data represent mean ± standard deviation of quadruplictate determinations. Bars with same letters are not significantly different at 5% level.

Figure 2 The effects of MEFS on cardiac and renal catalase activities in doxorubicin-induced oxidative stress in Wistar albino rats. Data represent Mean ± standard deviation of quadruplicate determinations. Bars with the same letters are not significantly different at 5% level.
Effects of MEFS on cardiac and renal glutathione level in doxorubicin oxidative stress in rats
The effects of MEFS on Cardiac and renal glutathione level in doxorubicim-induced oxidative damage are presented in Figure 3A and 3B respectively. Results show that doxorubicin administration significantly reduced (P<0.05) the concentration of glutathione in the cardiac and renal tissues. Pre-treatment of MEFS enhanced the level of both cardiac and renal glutathione concentrations. However, at 250mg/kg b.w of MEFS, cardiac glutathione concentration was significantly lower than that of the normal group. The extracts at all doses significantly raised renal glutathione level compared to doxorubicin groups.

Figure 3 The effects of MEFS on cardiac and renal glutathione concentrations in doxorubicin-induced oxidative stress in Wistar albino rats. Data represent Mean ± standard deviation of quadruplicate determinations. Bars with the same letters are not significantly different at 5% level.
Effects of MEFS on cardiac and renal malondialdehyde (MDA) in doxorubicin oxidative stress in rats
The effects of MEFS on cardiac and renal MDA are shown in Figure 4A and 4B respectively. Results show that cardiac and renal tissue MDA level were raised significantly (P<0.05) after doxorubicin administration. Pre-administration of MEFS reduced cardiac and renal MDA level. But in the renal tissue, 500mg/kg b.w MEFS was the most effective extract dose.

Figure 4 The effects of MEFS on cardiac and renal MDA in doxorubicin-induced oxidative stress in Wistar albino rats. Data represent Mean ± standard deviation of quadruplicate determinations. Bars with the same letters are not significantly different at 5% level.
Effects of MEFS on cardiac and renal histology in doxorubicin oxidative stress in rats
The effects of MEFS on cardiac and renal histology in doxorubicin-induced oxidative stress in rats are shown in Figure 5 and 6. Figure 5A shows normal cardiac tissue histology depicting cardiac myofibrils with homogenous diameter, forming a continuous meshwork called syncytium. The nuclei are centrally placed. Doxorubicin administration distorted cardiac muscle as evidenced by cardiac myofibril with heterogenous fibre diameter and peripherally placed nuclei (Figure 5B). Pre-administration of 250mg/kg b.w, 500mg/kg b.w and 750mg/kgb.w MEFS normalised the cardiac tissue histology characterised by normal cardiac myofibril with homogenous fiber diameter, centrally placed nuclei and continuous meshwork (Figure 5B-5C). Figure 6A shows the normal renal tissue histology, with normal renal tubules (RT) and glomeruli surrounded by patent bowman’s capsules (BC). Results show that administration of doxorubicin distorted the renal tissue histology characterised by glomerulus with occluded Bowman’s capsular spaces (Figure 6B). pre-administration of different doses of MEFS normalised the renal tissue histology (Figure 4C-4E).
Phytochemical analysis of MEFS revealed the presence of alkaloid, saponins, tannins glycoside in high amount but sterols in low amount. This is in agreement with Achi et al.22 However, the high saponin content observed in this study disagrees with Obonga et al.23 who actually reported low saponin content of the plant Ficus sur. The presence of these secondary metabolites in MEFS are responsible for the pharmacological properties reported for MEFS.
Natural products are not always safe as it is generally and incorrectly believed. Toxicity of natural products is well known.24 Thus, it is appropriate to conduct toxicity evaluation of natural products to reveal their inherent toxicities, and hence, to establish a safe working dose range for in vivo studies. In this study, acute toxicity study revealed that MEFS was practically non-toxic up to the dose of 5000mg/kg b.w., as no behavioural changes or death was observed (Table 3). This observation is in agreement with Eluka et al.10 who demonstrated the non-toxicity of MEFS. However, declaration of safety of MEFS may require sub-chronic and chronic toxicity evaluation to reveal its inherent toxic liabilities, most especially when the plant is intended for therapeutic purposes.
The effectiveness of doxorubicin in the treatment of wide range of solid and liquid tumours is well known in clinical medicine. However, doxorubicin is notorious for cardiotoxicity and nephrotoxicity after administration.1 The cardiotoxicity of doxorubicin ranges from perturbation of cardiac rhythm to congestive heart failure. In this study, administration of doxorubicin caused cardiotoxicity characterised by elevation of plasma troponin and Creatine kinase (CK-MB) (Table 4) and distortion of cardiac histological architecture (Figure 5B). These observations are in consonance with the report of cardiotoxicity of doxorubicin in clinical use.25 The poor antioxidant defence in the cardiac tissue, compared to other tissues, is presumed to render the cardiomyocytes more susceptible to the toxicity of redox cycling agent.26 Pre-administration of MEFS dose- dependently protected the cardiac tissues evidenced by reduction of elevated TnT and CK-MB and improvement of distorted cardiac tissue histology (Figure 5C-E) The extract was more effective at the dose of 500mg/kg b.w. The protective effects may be by induction of defence system in vivo or by direct scavenging of reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated by redox cycling doxorubicin.
The nephrotoxic effect of doxorubicin was demonstrated by significant elevation of plasma creatinine and urea concentrations (Table 5). Nephrotoxicity was also confirmed by alteration of renal histological architecture (Figure 6B). The nephroprotective effect of MEFS was demonstrated by significant reduction of the elevated creatinine and urea, and resolution of histological architecture of the kidneys (Figure 6C-E). Results also show that neither doxorubicin nor doses of MEFS had any significant effects on plasma electrolytes. Njoku et al.27 also demonstrated the cardioprotective effect of MEFS in carbon tetrachloride- induced toxicity via antioxidant mechanism. Since the toxicity of doxorubicin involve radical species, direct scavenging of the reactive species or modulation of redox system in vivo may be responsible for the observed protective effect of MEFS in this study. High phenolic content and potent radical scavenging ability of Ficus sur have been reported.23,27 Phenolics are generally known for induction of defence system in vivo.28 Additionally, pre-administration of substances with antioxidant properties have been shown to reduce the toxic effects of doxorubicin.29 Other phenolic rich extracts have been shown to ameliorate the toxicity of doxorubicin in experimental animals.30,31
Administration of Doxorubicin has been reported to decrease the activities of Catalase, Superoxide dismutase and glutathione peroxidase.7 The reduction of the activities of these enzymes may be due to decrease in protein synthesis. In this study, administration of Doxorubicin led to significant reduction in the activities of SOD and catalase in both cardiac and renal tissues (Figure 1 and Figure 2), where the toxicity of doxorubicin are always more pronounced.32 Results also show that pre-administration of MEFS enhanced the activities of cardiac and renal SOD most effectively at 500mg/kg b.w., but less effectively at the extract dose 750mg/kgbw. The restorative effects of MEFS on SOD and catalase activities may be due to radical scavenging or induction of the expression of the enzymes. This may lead to reduction of oxidative stress burden imposed by redox cycling doxorubicin. In fact, modulation of redox system is implicated in the anticarcinogenic33 and organ protective effects34 of flavonoids in certain toxicology models; hence, such protective mechanisms may be equally attributed to flavonoid-rich MEFS.
Glutathione (GSH) is an antioxidant as well as a cofactor for a number of enzymes, including glutathione peroxidase.35 In this study, administration of doxorubicin led to depletion of cardiac and renal GSH (Figure 5). This is in consonance with Thornally and Dod.36 The reduction in GSH concentration may be due to increased level of ROS and RNS that characterise doxorubicin administration.37 Pre-administration of MEFS significantly increased the cardiac and renal glutathione concentration. In the cardiac tissues, 250mg/kb b.w of MEFS was however less effective. However, all doses restored the concentration of glutathione in renal tissues. The preservation of renal and cardiac glutathione level may be due to direct antioxidant mechanism of phenolic components of MEFS and consequent reduction of oxidative stress burden in the tissues. This may have sparing effects on glutathione pool in vivo. Flavonoids are also inducers of glutathione synthesis by increasing the level of regulatory enzyme γ-glutamyl-cysteine synthatase.38 Bors et al.39 also demonstrated the enhancement of GSH concentration by phenolic- rich extract during oxidative stress.
Lipid peroxidation due to reactive specie accumulation is one of the hallmarks of doxorubicin administration.36 In this study, doxorubicin administration led to an increase in cardiac and renal MDA concentrations (Figure 4) confirming that lipid peroxidation occured. The observed increase in MDA is in consonance with Thornally and Dodd.36 In this study, results show that pre-administration of MEFS significantly reduced both cardiac and renal MDA, indicating that lipid peroxidation was prevented by MEFS. The reduction in lipid peroxidation by MEFS may be due to the antioxidant effect of the extract. Dogan et al.40 also reported the anti-lipid peroxidative effects of phenolic rich extract.
In this study, it is clear that MEFS is more effective at the dose of 500mg/kg b.w than 750mg/kgb.w in most cases. This suggests that MEFS may be less protective at a higher dose. This inverse dose-response effect, though difficult to interpret due to the complexity of plant extracts, may be due to pro-oxidant effects of phenolics at high concentration.41 At high concentration, flavonoids may autooxidise, generating ROS and RNS, thereby contributing to tissue damage.42,43 Protective effects of phenolic-rich plant extracts showing inverse dose response effects have been reported.44–47
From this study, it may be concluded that MEFS protected Wistar albino rats from toxicity of redox-cycling doxorubicin. The protective effects could be due to direct radical scavenging and/or modulation of redox system in vivo by MEFS. These antioxidant effects may have reduced oxidative burden imposed by doxorubicin, and hence, may be responsible for the observed protective effects of the MEFS methanolic extract in doxorubicin-induced oxidative damage in Wistar albino rats.
The authors wish to acknowledge the invaluable contributions of EngUchenna Obi, of Fordiac engineering plc, Port Harcourt, towards the execution of this research project.
None.
None.

©2022 Obi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.