Journal of
eISSN: 2473-0831


Research Article Volume 10 Issue 5
Department of Chemistry, Saurashtra University, India
Correspondence: Shipra Baluja, Department of Chemistry, Saurashtra University, Rajkot-360005 (Gujarat) India
Received: June 18, 2021 | Published: September 30, 2021
Citation: Baluja S. Evaluation of viscosity in binary mixtures of dimethyl sulphoxide at 298.15K. J Anal Pharm Res. 2021;10(5):169-175. DOI: 10.15406/japlr.2021.10.00383
The viscosity of binary mixtures of dimethyl sulphoxide with different alcohols such as methanol, ethanol, 1-propanol, iso-propanol, 1-butanol, iso-butanol, tertiary butanol has been determined at 298.15K. The experimental values are compared with theoretical values evaluated by different theories. It is observed that for some theories, values are in agreement with the experimental values. Further, an attempt has been made to study the intermolecular interactions in studied solutions in terms of excess free energy of mixing, strength of interaction parameters and interaction energy. The viscosity data of pure liquids and their mixtures are needed to design various chemical processes where heat and mass transfer are important.
Transport properties of binary solution have proven to be a useful tool in elucidating the interactions occurring in solutions. Viscosity is an important transport property.1 The determination of viscosity of liquid mixtures are of interest in chemical industries and chemical engineering involving fluid transportation, process design, pump operations, heat exchange, mixing agitation etc.2-9
In the present work, densities and viscosities of binary mixtures of dimethyl sulphoxide with some alcohols such as methanol, ethanol, 1-propanol, iso-propanol, 1-butanol, iso-butanol, ter-butanol have been measured at 298.15 K. For binary and non-electrolytic solutions, vvarious theories have been developed such as Bingham, Kendall-Munroe, Arrhenius- Eyring, Katti and Chaudhari, Grunberg-Nissan etc. These theories are tested for these studied binary mixtures using experimental data of viscosities. Further, some excess parameters such as excess volume and excess viscosities have also been evaluated.
The studied liquids used in the present study were of analytical grade and were supplied by S. D. Fine Chemicals Pvt. Mumbai. These liquids were purified by standard method.10 The purified liquids were stored in air tight bottles over 0.4 nm molecular sieves.
All the binary mixtures were prepared by volume and were stored in stoppered bottles to minimize the error due to evaporation. The densities of all the pure liquids and their binary solutions were measured by Anton Paar densitometer (model No. DSA 5000) at 298.15 K with accuracy of ±0.005 kg/m3. The viscosities of pure liquids and solutions were measured by Ubbleohde viscometer at 298.15 K. An electronic digital stop watch was used for the flow time measurements. The flow time measurements were done at least three times so that each data reproducible to ±0.05 s was obtained, and the results were averaged. The uncertainties in dynamic viscosities are of the order of ±0.003 mPa.s.
Theory
The viscosity deviations (Δη) are obtained by the equation:
where η12 is the viscosity of the mixture and x1, η1, x2 and η2 are the mole fraction and viscosity of pure components 1 and 2 respectively. Figure 1 shows the variation of viscosity deviation in studied solutions. It is evident from Figure 1 that as CH2 group in alcohol series increases, deviation increases and deviation is more in branched alcohols.
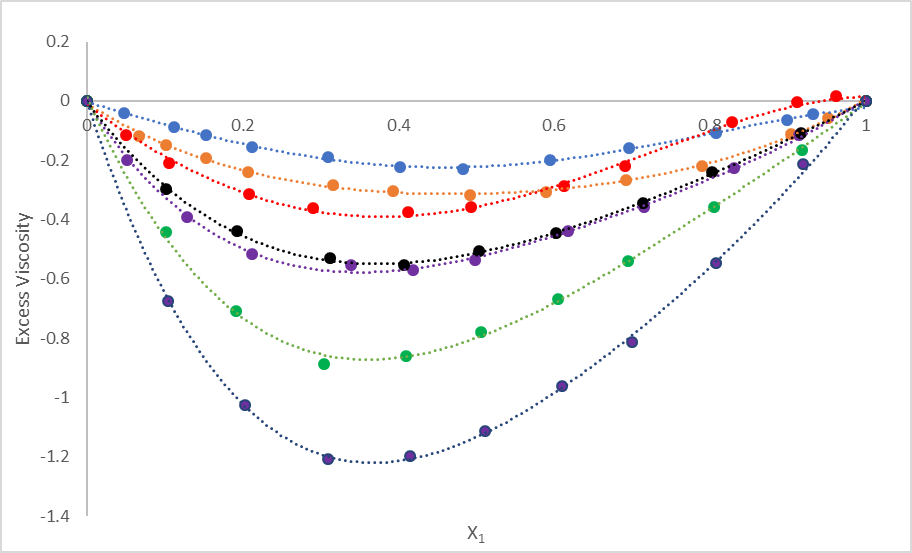
Figure 1 The variation of viscosity deviation for different binary solutions of DMSO with alcohols at 298.15K.
For binary liquid mixtures, ideal viscosity can be evaluated by the following equation proposed by Bingham:11
where xi and ηi are the mole fraction and viscosity of the i-component respectively.
The viscosity of binary solution can also be evaluated by an additive relation based on Arrhenius and Eyring model12 as follows:
Where Vi is the molar volume of the i-component.
Kendall and Munroe13 gave another equation for multi-component systems which is:
The following relation was proposed by Katti and Chaudhari14 for binary solutions:
where Wvis is the interaction energy parameter and is given by:
where V is the molar volume of the mixture and d is the Grunberg-Nissan interaction parameter, which is given by:
and
where ηexp is the experimental viscosity and ηBring is the viscosity calculated by Bringam relation.
The Grunberg-Nissan relation15 for viscosity of non-ideal mixture is given as:
The purity of the samples and accuracy of data were checked by comparing the measured densities and viscosities of the pure compounds with the literature values, which are given in Table 1. The experimental values are in agreement with literature values.
|
Liquid |
Density |
Viscosity |
|
Dimethyl sulphoxide |
1.0959 (1.0960)16 |
1.991 (1.991)10 |
|
Methanol |
0.7868 (0.7867)17 |
0.557 (0.553)19 |
|
Ethanol |
0.7854 (0.7855)18 |
1.074 (1.0825)20 |
|
Prop-1-ol |
0.7996 (0.7998)18 |
1.934 (1.943)10 |
|
Prop-2-ol |
0.7816 (0.78126)10 |
2.049 (2.0436)10 |
|
But-1-ol |
0.8067 (0.8059)18 |
2.568 (2.571)20 |
|
But-2-ol |
0.7987 (0.7998)10 |
3.333 (3.333)10 |
|
Ter-Butanol |
0.7809 (0.7812)10 |
4.439 (4.438)10 |
Table 1 Experimental Density and viscosity of pure liquids at 298.15 K along with literature values in parenthesis
The experimental values of viscosities for the studied binary solutions are given in Table 2.
|
x1 |
ηexp |
ΗBing |
ηArr |
ΗKen |
ΗKatti |
ΗGrun |
|
DMSO+Methanol |
||||||
|
0.0473 |
0.5853 |
0.6248 |
0.5886 |
0.5916 |
0.5492 |
0.5542 |
|
0.1114 |
0.6297 |
0.7167 |
0.6349 |
0.6419 |
0.5536 |
0.564 |
|
0.1528 |
0.6611 |
0.7761 |
0.6665 |
0.6767 |
0.5629 |
0.5765 |
|
0.2119 |
0.7065 |
0.8609 |
0.7173 |
0.7296 |
0.5813 |
0.5988 |
|
0.3098 |
0.8114 |
1.0013 |
0.8079 |
0.8265 |
0.6459 |
0.6698 |
|
0.4014 |
0.9114 |
1.1326 |
0.9042 |
0.9288 |
0.7185 |
0.7474 |
|
0.4824 |
1.0196 |
1.2487 |
1.0017 |
1.0298 |
0.808 |
0.8408 |
|
0.5949 |
1.2114 |
1.4101 |
1.1565 |
1.1885 |
0.984 |
1.0209 |
|
0.6956 |
1.3956 |
1.5545 |
1.3188 |
1.3512 |
1.1754 |
1.2129 |
|
0.8072 |
1.6052 |
1.7145 |
1.5314 |
1.5576 |
1.4256 |
1.458 |
|
0.8995 |
1.7828 |
1.8469 |
1.7356 |
1.752 |
1.6689 |
1.6908 |
|
0.932 |
1.8507 |
1.8935 |
1.8139 |
1.8261 |
1.7679 |
1.7844 |
|
DMSO+Ethanol |
||||||
|
0.0668 |
1.0155 |
1.1353 |
1.1197 |
1.1192 |
0.9991 |
1.0011 |
|
0.1009 |
1.0185 |
1.1665 |
1.1434 |
1.1431 |
0.9954 |
0.998 |
|
0.153 |
1.0213 |
1.2143 |
1.1809 |
1.1804 |
0.9895 |
0.9928 |
|
0.2062 |
1.0239 |
1.2631 |
1.2205 |
1.2198 |
0.9848 |
0.9888 |
|
0.3164 |
1.0792 |
1.3641 |
1.3064 |
1.3057 |
1.0278 |
1.0329 |
|
0.3926 |
1.1302 |
1.434 |
1.3692 |
1.3686 |
1.0728 |
1.0786 |
|
0.4913 |
1.2088 |
1.5245 |
1.4549 |
1.4546 |
1.1469 |
1.1532 |
|
0.5898 |
1.3088 |
1.6148 |
1.5459 |
1.5458 |
1.246 |
1.2527 |
|
0.6921 |
1.4408 |
1.7086 |
1.6472 |
1.6466 |
1.3817 |
1.3882 |
|
0.7894 |
1.5801 |
1.7978 |
1.7484 |
1.7485 |
1.5305 |
1.5364 |
|
0.9044 |
1.7904 |
1.9033 |
1.8765 |
1.8772 |
1.7612 |
1.7654 |
|
0.9518 |
1.8903 |
1.9468 |
1.932 |
1.9329 |
1.8733 |
1.8763 |
|
DMSO+Prop-1-ol |
||||||
|
0.0505 |
1.8161 |
1.9369 |
1.8926 |
1.9366 |
1.8143 |
1.8159 |
|
0.1049 |
1.7185 |
1.94 |
1.8971 |
1.9397 |
1.7167 |
1.7183 |
|
0.2086 |
1.6125 |
1.9459 |
1.9059 |
1.9455 |
1.6106 |
1.6122 |
|
0.2908 |
1.5593 |
1.9506 |
1.9133 |
1.9502 |
1.5574 |
1.559 |
|
0.4128 |
1.5396 |
1.9575 |
1.9254 |
1.9571 |
1.5376 |
1.5393 |
|
0.4932 |
1.5532 |
1.9621 |
1.9335 |
1.9617 |
1.5512 |
1.5529 |
|
0.6126 |
1.6192 |
1.9689 |
1.9457 |
1.9685 |
1.6171 |
1.6189 |
|
0.6914 |
1.6825 |
1.9734 |
1.9544 |
1.973 |
1.6804 |
1.6822 |
|
0.8277 |
1.8277 |
1.9812 |
1.9699 |
1.9808 |
1.8256 |
1.8274 |
|
0.9123 |
1.8896 |
1.986 |
1.9796 |
1.9857 |
1.8877 |
1.8893 |
|
0.962 |
1.9471 |
1.9888 |
1.9855 |
1.9886 |
1.9053 |
1.9068 |
|
DMSO+Prop-2-ol |
||||||
|
0.0516 |
1.848 |
2.046 |
2.0428 |
2.0463 |
1.8461 |
1.8478 |
|
0.1282 |
1.6498 |
2.0415 |
2.4266 |
2.0418 |
1.6478 |
1.6496 |
|
0.2124 |
1.5202 |
2.0366 |
2.031 |
2.0369 |
1.5181 |
1.52 |
|
0.3389 |
1.475 |
2.0293 |
2.0227 |
2.0295 |
1.4727 |
1.4748 |
|
0.4183 |
1.4551 |
2.0247 |
2.0181 |
2.0248 |
1.4527 |
1.4549 |
|
0.4989 |
1.4831 |
2.0201 |
2.0135 |
2.0202 |
1.4807 |
1.4828 |
|
0.618 |
1.5753 |
2.0131 |
2.0072 |
2.0133 |
1.5728 |
1.575 |
|
0.7156 |
1.6505 |
2.0075 |
2.0024 |
2.0077 |
1.648 |
1.6502 |
|
0.8309 |
1.7745 |
2.0008 |
1.9972 |
2.001 |
1.7721 |
1.7742 |
|
0.9141 |
1.8801 |
1.9959 |
1.9936 |
1.9962 |
1.878 |
1.8798 |
|
DMSO+But-1-ol |
||||||
|
0.1008 |
2.2114 |
2.5097 |
1.7351 |
2.5025 |
1.5283 |
2.205 |
|
0.192 |
2.017 |
2.457 |
1.75598 |
2.4451 |
1.4405 |
2.0072 |
|
0.3117 |
1.7572 |
2.3878 |
1.7538 |
2.3718 |
1.3867 |
1.8446 |
|
0.4075 |
1.7783 |
2.3325 |
1.8086 |
2.3147 |
1.3775 |
1.7645 |
|
0.5031 |
1.7721 |
2.2772 |
1.8328 |
2.2591 |
1.4256 |
1.7577 |
|
0.6021 |
1.7738 |
2.2199 |
1.8623 |
2.2029 |
1.4858 |
1.7597 |
|
0.7144 |
1.8117 |
2.1551 |
1.8939 |
2.1408 |
1.5912 |
1.7992 |
|
0.8032 |
1.8648 |
2.1038 |
1.9247 |
2.093 |
1.7025 |
1.8546 |
|
0.9174 |
1.9295 |
2.0377 |
1.9628 |
2.033 |
1.8548 |
1.9242 |
|
DMSO+But-2-ol |
||||||
|
0.1017 |
2.7536 |
3.1965 |
3.1498 |
3.1638 |
2.7139 |
2.7241 |
|
0.1917 |
2.366 |
3.0757 |
2.9998 |
3.0203 |
2.3088 |
2.3224 |
|
0.3048 |
2.0366 |
2.9239 |
2.8241 |
2.8494 |
1.9683 |
1.9839 |
|
0.4099 |
1.9219 |
2.7829 |
2.6724 |
2.6991 |
1.8467 |
1.8634 |
|
0.5056 |
1.874 |
2.6545 |
2.5426 |
2.5692 |
1.7962 |
1.8132 |
|
0.6044 |
1.8538 |
2.5218 |
2.4175 |
2.4416 |
1.7779 |
1.7942 |
|
0.6946 |
1.8599 |
2.4008 |
2.3102 |
2.3308 |
1.79 |
1.805 |
|
0.8047 |
1.8945 |
2.2531 |
2.1877 |
2.2022 |
1.8391 |
1.8511 |
|
0.9181 |
1.934 |
2.1009 |
2.0706 |
2.0772 |
1.9048 |
1.9116 |
|
DMSO+Ter-Butanol |
||||||
|
0.1038 |
3.5097 |
4.1847 |
4.065 |
4.086 |
3.41 |
3.4248 |
|
0.2023 |
2.9199 |
3.9435 |
3.7448 |
3.7756 |
2.7744 |
2.7939 |
|
0.3096 |
2.4754 |
3.6807 |
3.4236 |
3.4643 |
2.3072 |
2.3285 |
|
0.4154 |
2.2247 |
3.4216 |
3.1385 |
3.1825 |
2.0464 |
2.068 |
|
0.5113 |
2.073 |
3.1868 |
2.9029 |
2.9469 |
1.895 |
1.9159 |
|
0.6098 |
1.9855 |
2.9455 |
2.6845 |
2.723 |
1.815 |
1.8344 |
|
0.6994 |
1.9155 |
2.7261 |
2.5017 |
2.5342 |
1.7625 |
1.7796 |
|
0.8082 |
1.9131 |
2.4597 |
2.3003 |
2.3225 |
1.7918 |
1.8052 |
|
0.9198 |
1.9755 |
2.1864 |
2.1128 |
2.1236 |
1.9097 |
1.9174 |
Table 2 Experimental and theoretical viscosities in different binary solutions at 298.15 K
In Figure 1, deviation plots of excess viscosity are given for all the studied solutions. It is evident from Figure 1 that for all the solutions, values are negative. Further, at equimolar concentrations of studied solutions, the order is: methanol > ethanol > prop-1-ol > butan-1-ol > prop-2-ol > butan-2-ol > tertiary butanol. Thus, as CH2 group increases, interactions decrease which causes more negative values with higher or branched alcohols.
Using above relations, the theoretical viscosities and some thermodynamic parameters such as excess free energy of mixing, strength of interaction parameter and interaction energy etc., have been evaluated for all the studied binary mixtures. The theoretical velocities evaluated by Bingham, Arrhenius and Eyring model, Kendall and Munroe, Katti and Chaudhari and Grunberg-Nissan relations are also given in Table 1. The validity of these relations has been checked by calculating percentage deviations for all the studied binary liquid mixtures and the results are shown in Table 3. It is observed that for some binary solutions, some of the relations give better results than others. Further, in most of the solutions, deviations are higher in intermediate compositions. The limitations and approximations incorporated in these theories are responsible for the deviations. The deviations are different for different solutions for different theories. In DMSO + methanol system, there is good agreement between experimental and theoretical values evaluated by Arrhenius and Kendell relations. In all the other solutions except DMSO + But-1-ol, values evaluated by Katti and Grunberg relations are close to experimental values. In DMSO + But-1-ol solutions, experimental values are in good agreement with those evaluated by Grunberg relation. Additive property is considered in some theories, which gives better results in ideal solutions which is not the case in studied solutions. This is one of the reasons for discrepancy between experimental and theoretical values.
|
x1 |
ΗBing |
ηArr |
ΗKen |
ΗKatti |
ΗGrun |
|
DMSO+Methanol |
|||||
|
0.0473 |
-6.75 |
-0.56 |
-1.06 |
6.17 |
5.31 |
|
0.1114 |
-13.82 |
-0.82 |
-1.93 |
12.09 |
10.43 |
|
0.1528 |
-17.4 |
-0.81 |
-2.35 |
14.85 |
12.8 |
|
0.2119 |
-21.85 |
-1.53 |
-3.26 |
17.72 |
15.24 |
|
0.3098 |
-23.4 |
0.43 |
-1.86 |
20.4 |
17.45 |
|
0.4014 |
-24.27 |
0.79 |
-1.91 |
21.16 |
17.99 |
|
0.4824 |
-22.48 |
1.76 |
-1 |
20.75 |
17.54 |
|
0.5949 |
-16.4 |
4.53 |
1.89 |
18.77 |
15.72 |
|
0.6956 |
-11.39 |
5.51 |
3.18 |
15.78 |
13.09 |
|
0.8072 |
-6.81 |
4.6 |
2.96 |
11.19 |
9.17 |
|
0.8995 |
-3.59 |
2.65 |
1.73 |
6.39 |
5.16 |
|
0.932 |
-2.31 |
1.99 |
1.33 |
4.47 |
3.58 |
|
DMSO+Ethanol |
|||||
|
0.0668 |
-11.79 |
-10.22 |
-10.26 |
1.61 |
1.41 |
|
0.1009 |
-14.53 |
-12.23 |
-12.26 |
2.27 |
2.02 |
|
0.153 |
-18.9 |
-15.58 |
-15.63 |
3.12 |
2.79 |
|
0.2062 |
-23.36 |
-19.14 |
-19.2 |
3.81 |
3.43 |
|
0.3164 |
-26.4 |
-20.99 |
-21.05 |
4.77 |
4.29 |
|
0.3926 |
-26.88 |
-21.1 |
-21.15 |
5.08 |
4.57 |
|
0.4913 |
-26.12 |
-20.34 |
-20.36 |
5.12 |
4.6 |
|
0.5898 |
-23.38 |
-18.11 |
-18.12 |
4.8 |
4.29 |
|
0.6921 |
-18.59 |
-14.28 |
-14.32 |
4.11 |
3.65 |
|
0.7894 |
-13.78 |
-10.66 |
-10.65 |
3.14 |
2.77 |
|
0.9044 |
-6.31 |
-4.85 |
-4.81 |
1.63 |
1.4 |
|
0.9518 |
-2.99 |
-2.26 |
-2.21 |
0.9 |
0.74 |
|
DMSO+Prop-1-ol |
|||||
|
0.0505 |
-6.65 |
-4.22 |
-6.64 |
0.1 |
0.01 |
|
0.1049 |
-12.89 |
-10.39 |
-12.87 |
0.1 |
0.01 |
|
0.2086 |
-20.68 |
-18.2 |
-20.65 |
0.12 |
0.02 |
|
0.2908 |
-25.09 |
-22.7 |
-25.07 |
0.12 |
0.02 |
|
0.4128 |
-27.15 |
-25.06 |
-27.12 |
0.13 |
0.02 |
|
0.4932 |
-26.33 |
-24.49 |
-26.3 |
0.13 |
0.02 |
|
0.6126 |
-21.6 |
-20.17 |
-21.57 |
0.13 |
0.02 |
|
0.6914 |
-17.29 |
-16.16 |
-17.27 |
0.12 |
0.02 |
|
0.8277 |
-8.4 |
-7.78 |
-8.38 |
0.11 |
0.02 |
|
0.9123 |
-5.1 |
-4.77 |
-5.09 |
0.1 |
0.01 |
|
0.962 |
-4.29 |
-4.11 |
-4.27 |
0.1 |
0.01 |
|
DMSO+Prop-2-ol |
|||||
|
0.0516 |
-10.71 |
-10.55 |
-10.73 |
0.1 |
0.01 |
|
0.1282 |
-23.75 |
-47.08 |
-23.76 |
0.12 |
0.01 |
|
0.2124 |
-33.97 |
-33.6 |
-33.99 |
0.14 |
0.01 |
|
0.3389 |
-37.58 |
-37.14 |
-37.59 |
0.16 |
0.02 |
|
0.4183 |
-39.15 |
-38.69 |
-39.16 |
0.16 |
0.02 |
|
0.4989 |
-36.21 |
-35.77 |
-36.22 |
0.16 |
0.02 |
|
0.618 |
-27.8 |
-27.42 |
-27.81 |
0.16 |
0.02 |
|
0.7156 |
-21.63 |
-21.32 |
-21.64 |
0.15 |
0.02 |
|
0.8309 |
-12.75 |
-12.55 |
-12.77 |
0.13 |
0.02 |
|
0.9141 |
-6.16 |
-6.04 |
-6.18 |
0.11 |
0.01 |
|
DMSO+But-1-ol |
|||||
|
0.1008 |
-13.49 |
21.54 |
-13.17 |
30.89 |
0.29 |
|
0.192 |
-21.82 |
12.94 |
-21.23 |
28.58 |
0.49 |
|
0.3117 |
-35.89 |
5.57 |
-27.71 |
25.34 |
0.68 |
|
0.4075 |
-31.16 |
-1.7 |
-30.16 |
22.54 |
0.77 |
|
0.5031 |
-28.5 |
-3.42 |
-27.48 |
19.55 |
0.81 |
|
0.6021 |
-25.15 |
-4.99 |
-24.19 |
16.24 |
0.79 |
|
0.7144 |
-18.95 |
-4.54 |
-18.17 |
12.17 |
0.69 |
|
0.8032 |
-12.81 |
-3.21 |
-12.24 |
8.7 |
0.55 |
|
0.9174 |
-5.61 |
-1.73 |
-5.37 |
3.87 |
0.27 |
|
DMSO+But-2-ol |
|||||
|
0.1017 |
-16.09 |
-14.39 |
-14.9 |
1.44 |
1.07 |
|
0.1917 |
-30 |
-26.79 |
-27.66 |
2.42 |
1.84 |
|
0.3048 |
-43.57 |
-38.67 |
-39.91 |
3.35 |
2.59 |
|
0.4099 |
-44.8 |
-39.05 |
-40.44 |
3.91 |
3.05 |
|
0.5056 |
-41.65 |
-35.68 |
-37.1 |
4.15 |
3.25 |
|
0.6044 |
-36.04 |
-30.41 |
-31.71 |
4.1 |
3.21 |
|
0.6946 |
-29.08 |
-24.21 |
-25.32 |
3.76 |
2.95 |
|
0.8047 |
-18.93 |
-15.48 |
-16.24 |
2.92 |
2.29 |
|
0.9181 |
-8.63 |
-7.06 |
-7.4 |
1.51 |
1.16 |
|
DMSO+Ter-Butanol |
|||||
|
0.1038 |
-19.24 |
-15.82 |
-16.42 |
2.84 |
2.42 |
|
0.2023 |
-35.06 |
-28.25 |
-29.31 |
4.98 |
4.31 |
|
0.3096 |
-48.69 |
-38.31 |
-39.95 |
6.79 |
5.93 |
|
0.4154 |
-53.8 |
-41.08 |
-43.05 |
8.01 |
7.04 |
|
0.5113 |
-53.73 |
-40.03 |
-42.16 |
8.59 |
7.58 |
|
0.6098 |
-48.36 |
-35.21 |
-37.15 |
8.59 |
7.61 |
|
0.6994 |
-42.32 |
-30.61 |
-32.3 |
7.99 |
7.1 |
|
0.8082 |
-28.57 |
-20.24 |
-21.4 |
6.34 |
5.64 |
|
0.9198 |
-10.68 |
-6.95 |
-7.5 |
3.33 |
2.94 |
Table 3 Deviation between experimental and theoretical values of viscosity in studied binary solutions
Further, in studied binary solutions, some thermodynamic parameters such as interaction parameter d, interaction energy (WVis) and excess free energy of mixing (αFm) have also been evaluated for quantitative estimation of interactions in solutions. The evaluated values are listed in Table 4.
|
x1 |
d |
WVis |
αFm |
|
DMSO+Methanol |
|||
|
0.0473 |
-1.4503 |
-3806.76 |
161.9963 |
|
0.1114 |
-1.308 |
-3431.43 |
320.9581 |
|
0.1528 |
-1.239 |
-3232.61 |
397.5923 |
|
0.2119 |
-1.1833 |
-3121.24 |
489.8494 |
|
0.3098 |
-0.9833 |
-2595.82 |
521.1631 |
|
0.4014 |
-0.9044 |
-2371.6 |
538.6374 |
|
0.4824 |
-0.812 |
-2133 |
502.5606 |
|
0.5949 |
-0.6302 |
-1661.76 |
376.4692 |
|
0.6956 |
-0.5092 |
-1347.45 |
267.2746 |
|
0.8072 |
-0.4234 |
-1139.85 |
163.3234 |
|
0.8995 |
-0.3906 |
-1073.08 |
87.53749 |
|
0.932 |
-0.3607 |
-1004.54 |
56.65759 |
|
DMSO+Ethanol |
|||
|
0.0668 |
-1.7883 |
-4530.75 |
276.3306 |
|
0.1009 |
-1.4958 |
-3787.71 |
336.3721 |
|
0.153 |
-1.3357 |
-3383.97 |
429.0644 |
|
0.2062 |
-1.2826 |
-3248.79 |
520.3998 |
|
0.3164 |
-1.0833 |
-2749.76 |
580.7955 |
|
0.3926 |
-0.9984 |
-2536.73 |
590.1648 |
|
0.4913 |
-0.9285 |
-2360.01 |
575.217 |
|
0.5898 |
-0.8685 |
-2210.03 |
520.8725 |
|
0.6921 |
-0.8001 |
-2044.95 |
422.6595 |
|
0.7894 |
-0.7767 |
-1984.54 |
320.065 |
|
0.9044 |
-0.7075 |
-1817.91 |
151.6255 |
|
0.9518 |
-0.642 |
-1667.05 |
73.00559 |
|
DMSO+Prop-1-ol |
|||
|
0.0505 |
-1.3428 |
-2185.26 |
159.602 |
|
0.1049 |
-1.2911 |
-2637.89 |
300.496 |
|
0.2086 |
-1.1384 |
-2528.24 |
465.8539 |
|
0.2908 |
-1.0856 |
-2474.09 |
554.9766 |
|
0.4128 |
-0.9908 |
-2300.44 |
595.3148 |
|
0.4932 |
-0.935 |
-2185.38 |
579.3108 |
|
0.6126 |
-0.824 |
-1932.64 |
484.7382 |
|
0.6914 |
-0.7475 |
-1755.28 |
395.3273 |
|
0.8277 |
-0.5654 |
-1322.63 |
199.8764 |
|
0.9123 |
-0.6219 |
-1475.11 |
123.341 |
|
0.962 |
-1.148 |
-2798.29 |
104.0231 |
|
DMSO+Prop-2-ol |
|||
|
0.0516 |
-2.0799 |
-5132.61 |
-1778.19 |
|
0.1282 |
-1.9063 |
-8585.42 |
252.3096 |
|
0.2124 |
-1.7484 |
-4313.93 |
528.1429 |
|
0.3389 |
-1.4241 |
-3511.91 |
725.0038 |
|
0.4183 |
-1.3577 |
-3349.25 |
790.8784 |
|
0.4989 |
-1.236 |
-3048.65 |
818.9175 |
|
0.618 |
-1.0389 |
-2561.89 |
765.9417 |
|
0.7156 |
-0.9621 |
-2373.26 |
607.95 |
|
0.8309 |
-0.8543 |
-2109.58 |
485.3763 |
|
0.9141 |
-0.7617 |
-1887.21 |
297.5384 |
|
DMSO+But-1-ol |
|||
|
0.1008 |
-1.39666 |
-3472.25 |
313.8001 |
|
0.192 |
-1.27255 |
-3165.29 |
489.3629 |
|
0.3117 |
-1.17202 |
-2714.69 |
623.2957 |
|
0.4075 |
-1.12425 |
-2795.98 |
672.8568 |
|
0.5031 |
-1.00406 |
-2491.83 |
622.1981 |
|
0.6021 |
-0.93769 |
-2337.45 |
556.8618 |
|
0.7144 |
-0.85228 |
-2116.81 |
431.0472 |
|
0.8032 |
-0.76517 |
-1924.26 |
299.8121 |
|
0.9174 |
-0.72623 |
-1852.42 |
136.4142 |
|
DMSO+But-2-ol |
|||
|
0.1017 |
-1.6326 |
-4042.72 |
369.7236 |
|
0.1917 |
-1.6931 |
-4189.18 |
650.3041 |
|
0.3048 |
-1.7068 |
-4223.87 |
896.4812 |
|
0.4099 |
-1.5304 |
-3788.31 |
917.6201 |
|
0.5056 |
-1.3929 |
-3446.85 |
863.0638 |
|
0.6044 |
-1.2872 |
-3186.68 |
762.9138 |
|
0.6946 |
-1.2035 |
-2981.62 |
632.8395 |
|
0.8047 |
-1.103 |
-2738.01 |
429.7003 |
|
0.9181 |
-1.1009 |
-2752.25 |
205.1969 |
|
DMSO+Ter-Butanol |
|||
|
0.1038 |
-1.89143 |
-4683.2 |
436.1524 |
|
0.2023 |
-1.86267 |
-4607.98 |
745.1024 |
|
0.3096 |
-1.85644 |
-4577.46 |
983.6187 |
|
0.4154 |
-1.7733 |
-4366.07 |
1067.46 |
|
0.5113 |
-1.72164 |
-4231.91 |
1066.361 |
|
0.6098 |
-1.65858 |
-4078.38 |
978.2627 |
|
0.6994 |
-1.67987 |
-4130.68 |
875.4577 |
|
0.8082 |
-1.62342 |
-3996.29 |
623.7957 |
|
0.9198 |
-1.38082 |
-3397.3 |
252.4923 |
Table 4 Evaluated thermodynamic parameters for the studied binary solutions at 298.15K
It is evident from Table 4 that for all the studied solutions, interaction parameter (d) values are negative which suggest weak interactions in these solutions.21 This is further supported by interaction energy which is again negative for these solutions. αFm is a measure of excess free energy of mixing. For studied solutions, these values are positive. Thus, excess free energy of mixing is reverse of interaction parameter and interaction energy. The negative αFm suggests strong interaction between molecules whereas positive values are due to weak interactions. Thus, in the studied solutions, weak interactions exist. This is further confirmed by negative excess viscosity which is shown in Figure 1.
Thus, it concluded that in studied binary solutions, some theories require modifications to give better results. Further, in studied solutions, weak interactions between molecules exist.
None.
The author declares there is no conflict of interest.

©2021 Baluja. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.