Journal of
eISSN: 2473-0831


Research Article Volume 6 Issue 5
Correspondence: Abdalla Ahmed Elbashir, Department of Chemistry, Faculty of science, University of Khartoum, Sudan
Received: August 01, 2017 | Published: December 21, 2017
Citation: Osman RAM, Elbashir AA (2017) Development and Validation of Stability Indicating HPLC Method for the Simultaneous Analysis of Amlodipine, Hydrochlorothiazide and Valsartan in Pharmaceutical Formulation. J Anal Pharm Res 6(5): 00188. DOI: 10.15406/japlr.2017.06.00188
A simple, specific, accurate and precise, stability indicating HPLC method for the simultaneous determination of antihypertensive drug combination consisting of amlodipine (AML), hydrochlorothiazide (HCT) and valsartan (VAL) was developed and validated. The optimized conditions for the separation of the three analytes was consisted of ODS C-18 (250mm×4.6mm i.d. 5μm) column, mobile phase: acetonitrile-potassium dihydrogen phosphate pH 3.5- acetonitrile (45:55% v/v). Column temperature was maintained at 40oC; mobile phase flow rate: 1.5mL/min; wave length : 230nm; and injection of 20μL. The precision of the method was measured through adequate repeatability or intraday precision (RSD ≤ 2) and interday precision (RSD ≤ 2). The method demonstrated adequate linearity of detector response over the range of 0.5-250%. The limits of detection for AML, HCT and VAL were 0.011, 0.010 and 0.010µg/mL, while the limits of quantification were 0.032, 0.020 and 0.019µg/mL respectively. The method also showed adequate robustness to variations in mobile phase, pH, and column temperature and acetonitrile concentrations. The full recoveries of each working standard for all compounds were within ICH specifications of 98-101% which showed that the method was accurate. The proposed method proved to be stability indicating by resolution of the analytes from their accelerated storage conditions products. The developed method is rapid (run time 8min), selective, requires simple sample preparation procedures and simple mobile phase combinations. It is also cost effective and represents a good procedure for determination of AML, HCT and VAL in bulk raw materials and pharmaceutical dosage forms.
Keywords:column temperature, sample preparation, blood pressure, hypertension, acetonitrile
Hypertension or high blood pressure, sometimes called arterial hypertension, is a chronic medical condition in which the blood pressure in the arteries is elevated. This requires the heart to work harder than normal to circulate blood through the blood vessels. The main factor that characterizes a rational drug combination is a synergistic action without side effects. In 2009, the US Food and Drug Administration (FDA) and the European Medicines Agency approved a triple fixed-dose combination of AML, HCT and VAL. It was found that the use of this triple combination was generally more effective in reducing blood pressure and providing overall blood pressure control than the dual combination therapies regardless of age, race, gender, ethnicity, or hypertension severity.1,2
Amlodipine besylate (AML) is chemically described as 3-Ethyl-5- methyl (±)-2-(2-aminoethoxy) methyl-4-(2-chlorophenyl)-l, 4-dihydro-6-methyl-3, 5- pyridinedicarboxylate, monobenzenesulphonate (Figure 1A). AML tablets are formulated as white tablets equivalent to 2.5, 5, and 10mg of AML for oral administration. AML is formulated as the besylate salt of AML, a long-acting calcium channel blocker.3
Hydrochlorothiazide (HCT) is a white, practically odorless crystalline powder, slightly soluble in water, sparingly soluble in alcohol and soluble in acetone (pKa 7.0). HCT is chemically described as 6-chloro-1,1-dioxo-3, 4-dihydro-2H-1, 2, 4-benzothiadiazine-7-sulfonamide (Figure 1B).4−6
Valsartan (VAL) is a white fine powder. It is soluble in ethanol and methanol but slightly soluble in water. Its melting point is 117oC. VAL is available as tablets for oral administration, containing 40, 80, 160 or 320mg of the drug. VAL is a nonpeptide, orally active and specific angiotensin II receptor blocker acting on the AT1 receptor subtype. VAL is chemically described as N-(1-oxopentyl)-N-[2'-(1H-tetrazol-5-yl) [1, 1’-0biphenyl-4-yl] methyl]-L-valine (Figure 1C).
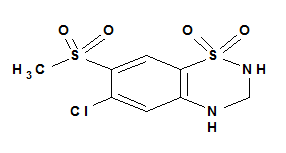
1A: HCT.
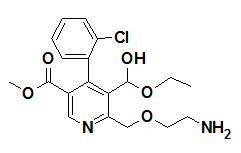
1B: AML.
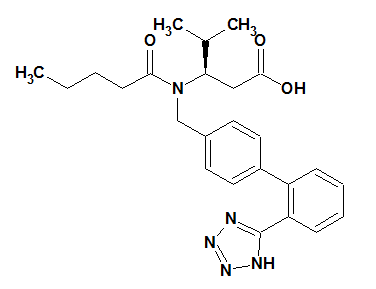
1C: VAL
Figure 1 Chemical structures of amlodipine (AML), hydrochlorothiazide (HCT) and valsartan (VAL).
Literature survey revealed that a number of methods have been reported for estimation of AML, HCT and VAL individually or in combination with other drugs. However, there is very few analytical methods reported for the simultaneous analysis of these drugs in a combined dosage formulation. The reported methods are UV spectrophotometric,7−12 spectrofluorimetric13 HPLC and HPTLC,14−25 capillary electrophoresis26−29 and electrochemical 30−32 methods.
Stability testing is an important issue in the process of drug product development. The purpose of stability testing is to provide evidence on how the quality of a drug substance or drug product varies with time under the influence of a variety of environmental factors, such as temperature, humidity, and light, and enables recommendation of storage conditions, retest periods, and shelf lives to be established. The two main aspects of a drug product that play an important role in shelf life determination are assay of the active drug and degradation products generated during the stability study. The drug product in a stability test sample needs to be determined using a stability indicating method, as recommended by the International Conference on Harmonization (ICH) guidelines33 and U.S. Pharmacopia (USP).34 Although stability indicating methods have been reported for assay of various drugs in drug products, most of them describe assay procedures for drug products containing only one active drug substance. To best of our knowledge no HPLC stability indicating method for the simultaneous determination of AML, HCT and VAL was reported. Therefore the aim of this work is to develop stability indicating HPLC method for the simultaneous determination of AML, HCT and VAL in mixtures without the need of prior separation step.
Materials
AML, HCT and VAL working standards were obtained from Azal pharmaceutical industries company Ltd, (Khartoum, Sudan). Acetonitrile HPLC grade, Methanol HPLC grade, potassium dihydrogen orthophosphate AR was purchased from Scharlau chemical (Spain). High purity deionised water was obtained from Azal pharmaceutical (Khartoum, Sudan) purification system.
Methods
Instrumentation
Chromatographic separation was performed with Shimadzu high performance liquid chromatography having C18, ODS (250mm×4.6mm), 5.0μm analytical column with photodiode array detector provided by Auto sampler and the Chromatographic data were recorded by LC Solution software. Absorbance was carried out by using UV-visible spectrophotometer model Shimadzu 1800 with quartz cells of 1cm optical path length, pH meter was used for pH measurements, analytical balance and ultrasonic bath.
Chromatographic conditions
For HPLC a number of preliminary trials were conducted with combinations of different organic solvents, compositions, and flow rate to check the retention time, shape, resolution, and other chromatographic parameters. Among all tried experiments, the mobile phase combination is potassium dihydrogen orthophosphate AR (0.05M) in water:acetonitrile (45:55%v/v) adjust to pH 3.5. The solution was degassed in an ultrasonic water bath for 5minutes and filtered through 0.45μm nylon filter. The instrumental settings flow rate of 1.5mL/min; the column temperature is 40°C, and detector wavelength is 230nm found to be most suitable.
Preparation of standard and sample solutions
Stock standard solutions of AML, HCT and VAL (200, 200 and 250μg/mL) respectively: An accurately 20,20 and 25mg of AML, HCT and VAL standards were dissolved in mobile phase , transferred into 100mL volumetric flask, diluted with same solvent and mixed well.
Standard solutions of AML, HCT and VAL (40, 40 and 50μg/mL): The standard solutions were prepared by dilution of the stock standard solution with mobile phase to reach a concentration range 0.20-80μg/mL for AML, 0.2-80μg/mL for HCT and 0.25-100μg /mL for VAL. Triplicate 20μL injections were made for each concentration and chromatographed under the condition described above. The peak area of each concentration was plotted against the corresponding concentration to obtain the calibration graph and regression equation was computed.
Assay of commercial tablets
Ten Exforge HCT tablets were weighed and their mean was determined. After grinding the tablets into a fine powder in glass mortar, an accurately weighed quantity of the tablet powder equivalent 5mg AML, 12.5mg HCT and 160mg VAL the solution was stirred for 10min then diluted with mobile phase into a 100-mL volumetric flask. The sample solution was then filtered using 0.45μm filters (Millipore, Milford, MA).
Stability study
The samples were kept in the stability chamber (Thermaolab) set at accelerated storage conditions38 of 40±2°C and 75±5% relative humidity and in real storage conditions of 25±2°C and 60% relative humidity. The samples were periodically monitored by the HPLC analysis. Analyses were carried out at time 0 and after storage of 1, 2, and 3months in the stability chamber.
Chromatography optimization
The chromatographic conditions were optimized to achieve the best resolution and peak shape for AML, HCT, and VAL. Different mobile phases containing acetonitrile in phosphate buffer were examined. With acetonitrile content 45% or less, the retention time for VAL was far (retention time >10min) and the resolution between the AML and HCT less than 2.5, with decreasing of acetonitrile more than 40% the retention time of VAL too far up to 15min eluted. On the other hand to give short time increased the acetonitrile to 60%, the retention time for valsartan was short (retention time < 5.5min) but the resolution between the AML and HCT less than 2.0, moreover phosphate buffers of pH 3.0-7.0 were tried but the peak shapes for the drugs were sufficiently symmetrical only for pH value below 4 and the resolution warmed over. Therefore, the mobile phase containing acetonitrile and phosphate buffer at pH 3.5 (55:45, v/v) was selected as optimal for obtaining well defined and resolved peaks with mean retention times of 1.7 , 2.2 and 6.0 min, for standard solution HCT, AML and VAL, respectively, as shown in Figure 2.

Figure 2 Chromatogram of the standard solutions of HCT, AML and VAL respectively at optimized HPLC conditions.
Validation of the method
Method validation is the process of determining, through laboratory studies, that the performance characteristics of the method meet the requirements for its intended analytical applications. The characteristics that are studied during method validation include sensitivity, accuracy, precision, specificity, linearity of detector response, range of analyte concentration and robustness.
Specificity
The specificity of the HPLC method was ascertained by analyzing standard drug and sample solution .The retention time of AML, HCT and VAL were confirmed by comparing the retention time with that of standard.
Linearity
The linearity of the proposed HPLC procedure was evaluated by analyzing a series of different concentrations for each of the three analytes. The linear regression equations were generated by least squares treatment of the calibration data. Under the optimized conditions described above, the measured peak areas were found to be proportional to concentrations of the analytes. Table 1 presents the performance data and statistical parameters including linear regression equations, concentration ranges, and correlation coefficients. The data obtained from the linearity determination experiments was subjected to linear regression analysis. The linearity equations were y = 23,447.2x +3,996.1, R2 = 0.9996 for Amlodipine
y = 37,204.5x + 66,638.8, R2= 0.9993 for hydrochlorothiazide and
y = 40,039.99x + 22,825 R2= 0.9993 for valsartan. Correlation coefficient R2 values were in all cases greater than 0.999 indicating a strong correlation between the concentrations of the analytes and the peak areas and therefore the method could be applied in the assay of all or any of the three analyte compounds. A summary of the linearity analysis results obtained is shown in Table 1. The ICH guidelines recommend that for the establishment of linearity a minimum of five concentrations be utilized over the range of 80 to 120%.40, 41 This method was found to be linear over the range tested for all the three compounds.
|
Parameter |
AML |
HCT |
VAL |
|
Measurement wavelength (nm) |
230 |
230 |
230 |
|
Linear range (μg/mL) |
0.2 - 80 |
0.2 - 80 |
0.25 - 100 |
|
Standard deviation of the Blank |
74.9 |
74.9 |
74.9 |
|
Intercept |
3,996.10 |
66,639 |
22,824.90 |
|
Slope |
23,447.10 |
37,204.50 |
40,040 |
|
Correlation coefficient (r) |
0.99996 |
0.9993 |
0.9993 |
|
Limit of detection, LOD (μg/mL) |
0.011 |
0.01 |
0.01 |
|
Limit of quant., LOQ (μg/mL) |
0.032 |
0.02 |
0.019 |
Table 1 Parameters of Linearity data for the Amlodipine (AML), Hydrochlorothiazide (HCT), and Valsartan (VAL)
Accuracy
The accuracy of an analytical method is the closeness of the test results obtained by that procedure to a true known value. The accuracy for the developed method was determined by spiking the finished commercial products with working standards of compounds under study. The difference between spiked sample result and the unspiked sample resulted was calculated as percentage of the known added spike concentration. For this purpose one commercial compound that contained all the 3 compounds was used. The standards were added to the samples at 3 concentrations, 50, 100 and 150% of the assay concentrations and injected in triplicate. The standards were added at amounts that would increase the concentration by 20%. The % recovery of each added working standard was regarded as the accuracy (Table 2). The recoveries of all compounds were within the specified guidelines (ICH) of 98-103%.33, 34
|
Sample No |
Sample Content (μg/ mL) |
Average of Amount Found (μg/ mL) |
Assay % |
Recovery % |
RSD % |
Average of Recovery and RSD |
||
|
|
|
|
(Relative to 100% Concentration ) |
|||||
|
Amlodipine |
||||||||
|
AML |
20 |
19.73 |
49.325 |
98.65 |
0.41 |
99.55% |
||
|
40 |
39.88 |
99.7 |
99.7 |
0.17 |
0.23% |
|||
|
60 |
60.18 |
150.45 |
100.3 |
0.12 |
||||
|
HCT |
||||||||
|
HCT |
20 |
19.74 |
49.35 |
98.7 |
0.21 |
99.52% |
||
|
40 |
39.88 |
99.7 |
99.7 |
0.22 |
0.18% |
|||
|
60 |
60.09 |
150.23 |
100.15 |
0.12 |
||||
|
Valsartan |
||||||||
|
VAL |
25 |
24.88 |
49.76 |
99.52 |
0.15 |
100.01% |
||
|
50 |
49.99 |
99.98 |
99.98 |
0.17 |
0.15% |
|||
|
|
75 |
75.41 |
150.82 |
100.55 |
0.13 |
|
||
Table 2 Recovery studies for the determination of Amlodipine (AML), Hydrochlorothiazide (HCT), and Valsartan (VAL) by the proposed method
Precision
The precision of an analytical procedure as the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. Precision may be considered at three levels: repeatability, intermediate precision and reproducibility. The ICH5 requires repeatability to be tested from at least six replications measured at 100 percent of the test target concentration or from at least nine replications covering the complete specified range.and calculation the relative standard deviation for various assays done on same day or different days. The results obtained are summarized in Table 3.34,35 The relative standard deviation in both cases was below 2% indicating that the method was precise.
|
Change In |
Before Change |
Flow Rate |
Flow Rate |
Mobile Phase |
Mobile Phase |
λ nm |
λ nm |
|
Parameter |
1.4ml/min |
1.6 ml/min |
+10% acetonitrile |
-10% acetonitrile |
-5nm (225) |
+5nm (235) |
|
|
Amlodipine |
|||||||
|
Retention Time |
2.133 |
2.374 |
2.095 |
2.005 |
3.096 |
2.234 |
2.233 |
|
Tailing Factor |
1.277 |
1.288 |
1.272 |
1.276 |
1.288 |
1.275 |
1.277 |
|
Hydrochlorothiazide |
|||||||
|
Retention Time |
1.694 |
1.809 |
1.588 |
1.322 |
1.83 |
2.234 |
1.69 |
|
Tailing Factor |
1.222 |
1.247 |
1.244 |
1.212 |
1.234 |
1.223 |
1.244 |
|
Valsartan |
|||||||
|
Retention Time |
6.332 |
6.939 |
6.129 |
6.122 |
10.526 |
6.534 |
6.521 |
|
Tailing Factor |
1.16 |
1.252 |
1.121 |
1.172 |
1.234 |
1.18 |
1.19 |
Table 3 Influence of small variation in the assay condition on the analytical performance of the Proposed HPLC method for determination of Amlodipine (AML), Hydrochlorothiazide (HCT) and Valsartan (VAL).
Sensitivity
Limit of detection: The limit of detection (LOD) is the lowest amount of analyte in a sample that can be detected but not necessarily quantified using an analytical technique under specified experimental conditions. The LOD was established by signal over noise (S/N) method where 6 injection of blank (mobile phase) were injected and the mean peak height was determined. Determination of LOD is indicated for method developed for determination of restricted substances and degradation products that are usually present in small quantities in the sample34 while the LOQ was determined for this method to give an idea of the amount of each compound which can be quantified with adequate precision and accuracy to enable the profiling of related substances and degradation products during stability studies. The low limit of detection indicates that the developed method can be used for detection of low concentration of active constituents extracted from samples (Table 1).
Limit of quantification: The limit of quantification (LOQ) is the lowest amount of analyte in a sample that can be determined with acceptable precision under specified experimental conditions. The degree of precision considered to be acceptable for purposes of LOQ determination from peak areas of six injections, the low limit of quantification indicates that the developed method can be used for quantification of low concentration of active constituents extracted from samples (Table 1).
Robustness
The robustness of an analytical procedure is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters and provides an indication of its reliability during normal usage.34 In liquid chromatography variations of pH in mobile phase, mobile phase composition, column, temperature and flow rate are considered. Robustness of the method was determined from the degree of variation observed in peak areas and retention times from the same working standard solution analyzed while adjusting each of the liquid chromatographic factors indicated. Six replicate injections of the same working standard solution were run after having adjusted a single chromatographic parameter and coefficient of variation of peak areas of component peaks calculated. The degree of variation observed was then used to infer the method’s robustness Table 4.
|
Sample No |
Intraday Precision |
Interday Precision |
||
|
Assay % |
RSD % |
Recovery Mean |
RSD % |
|
|
Amlodipine |
||||
|
1 |
99.96 |
0.27 |
99.94 |
0.36 |
|
2 |
99.4 |
0.23 |
98.1 |
0.32 |
|
3 |
99.81 |
0.34 |
99.34 |
0.29 |
|
Average |
99.72 |
0.28 |
99.13 |
0.32 |
|
All assay |
99.43 |
RSD % |
0.42 |
|
|
Hydrochlorothiazide |
||||
|
1 |
98.76 |
0.31 |
98.3 |
0.12 |
|
2 |
98.24 |
0.22 |
99.87 |
0.18 |
|
3 |
98.55 |
0.27 |
99.23 |
0.31 |
|
Average |
98.52 |
0.27 |
99.13 |
0.2 |
|
All assay |
98.83 |
RSD % |
0.44 |
|
|
Valsartan |
||||
|
1 |
98.86 |
0.33 |
99.84 |
0.18 |
|
2 |
101.76 |
0.24 |
99.05 |
0.13 |
|
3 |
99.45 |
0.45 |
99.43 |
0.34 |
|
Average |
100.02 |
0.34 |
99.44 |
0.22 |
|
All assay |
|
99.73 |
RSD % |
0.41 |
Table 4 Precision studies for the determination of Amlodipine (AML), Hydrochlorothiazide (HCT), and Valsartan (VAL) by the proposed method
Stability tests
The stability test results in terms of the average content of AML, HCT, and VAL per tablet, in percentage with respect to the labeled amount, are presented in Table 5. The quantity of AML, HCT, and VAL remained fairly constant in accelerated and real storage until the end of the stability study period (3months). The stability results suggest that No significant degradation was observed and the relative standard deviation between the result of time initial and after 1, 2, and 3months in the stability chamber.
|
Time (Month) |
Accelerated Storage Conditions |
Real Storage Conditions |
||||
|
40°C and 75% |
25°C and 60% |
|||||
|
|
AML % |
HCT % |
VAL % |
AML % |
HCT % |
VAL % |
|
0 Time |
99.72 |
99.13 |
100.02 |
99.72 |
99.13 |
100.02 |
|
1 month |
99.2 |
100.02 |
98.8 |
101 |
98.6 |
99.47 |
|
2 month |
99.4 |
100.2 |
99.55 |
98.74 |
100.22 |
99.31 |
|
3 month |
99.14 |
99.57 |
99.93 |
98.9 |
99.34 |
99.56 |
Table 5 Stability studies for the determination of Amlodipine (AML), Hydrochlorothiazide (HCT), and Valsartan (VAL) by the proposed method
Analysis of pharmaceutical dosage form
The optimized HPLC procedure was applied for the assay of this drug combination in the pharmaceutical formulation (Exforge HCT tablets). The active ingredients were extracted with the same solvent used for the preparation of the standard stock solutions (HPLC-grade acetonitrile and phosphate buffer 55%:45%) then dilution was made with same solvent to reach concentration levels within the specified ranges. The active ingredients eluted at their specific retention times as shown in Figure 3. No interfering peaks were observed from any of the inactive ingredients or the colored coat of the analyzed tablets. The diode-array detection enables peak purity verification where no signs of co-elution from any of the inactive adjutants were detected. Recoveries were calculated using both external standard and standard addition methods. The assay results revealed satisfactory accuracy and precision as indicated from% recovery, SD and RSD% values Table 2 & 4. Although no monograph for the sample combinations are present in official pharmacopoeia, the assay limits specified in the British Pharmacopoeia and United States Pharmacopoeia for single component containing any of the three ingredients were used as a basis for determining whether the products met quality specifications. In all the three cases, the pharmacopoeia specified assay limits of 90-110% for each drug component3,36 (Table 4). It is evident from these results that the proposed method is applicable to the assay of this drug combination with a satisfactory level of selectivity, accuracy and precision.
In this study a method for the simultaneous determination of Amlodipine (AML), hydrochlorothiazide (HCT) and valsartan (VAL) an antihypertensive fixed dose combination in the market was developed. The optimum conditions for the separation of the AML, HCTand VAL was determined using isocratic reversed phase HPLC method by using ODS C-18 (250mm×4.6mm i.d. 5μm) column because these types of columns are the ones routinely and cheaply used in liquid chromatography analysis of pharmaceuticals. And a mobile phase consisting of acetonitrile-0.05 M KH2PO4 pH 3.0 (55:45 % v/v). Column temperature was maintained at 40°C and the flow rate was 1.5ml/min. Detection was at 230nm and injection of 20μL. All components eluting within 6min. The order of elution of the component was HCT, AML and VAL respectively. The % recovery was found to be within limits of the acceptance criteria with recovery range 98.6 to 100.3% for AML, 98.7 to 100.1% for HCT and 99.5 to 100.5% for VAL. The high percentage of recovery indicates that the proposed method is highly accurate. The detection limit of the proposed method was 0.011μg/mL, 0.10μg/mL and 0.10μg/mL and the quantification limit was 0.032μg/mL, 0.019μg/mL and 0.020μg/mL for AML, VAL and HCT respectively, which indicate the sensitivity of the method. The assay procedures were repeated more times and the results were found to give 99.43%, 98.83%and 99.73% for AML, HCT and VAL respectively. The number of theoretical plates calculated was 3019, 2404 and 6094 for AML, VAL and HCT respectively, which indicates efficient performance of the column. No interfering peaks were found in the chromatogram of the formulation within the run time indicating that excipients used in tablet formulations did not interfere with the simultaneous estimation of the drugs HCT, VAL and AML by the proposed HPLC method. The study focused on optimization of the conditions for simple, rapid and cost effective analysis including selection of routinely used columns and mobile phases to obtain satisfactory results.
The present paper described the development of simple, sensitive and accurate analytical methods for the simultaneous determination of AML, HCT and VAL in mixtures without the need of prior separation step. The procedure presented here does not need any expensive apparatus; therefore the proposed method can be used advantageously as a routine method for the determination of AML, HCT and VAL in quality control and industry, our method may be applied to the determination the content of the cited drugs in commercial tablets.
None.
None.
None.

©2017 Osman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.