Journal of
eISSN: 2473-0831


Research Article Volume 7 Issue 5
Correspondence: Hassouna MEM, Chemistry Department, Faculty of Science 62514, Beni-Suef University, Egypt, Tel +2 01223861504, Fax -803
Received: October 06, 2018 | Published: October 18, 2018
Citation: Hassouna MEM, Mohamed MA. Development and validation of RP-HPLC method for determination of amoxicillin residues and application to NICOMAC coating machine. J Anal Pharm Res. 2018;7(5):586-594. DOI: 10.15406/japlr.2018.07.00287
The Cleaning Validation protocol plays an important role in the field of pharmaceutical industries; its main task is the verification of cleaning procedures to ensure that complete removal of product residues, degradation products, preservatives, excipients, cleaning agents and cross-contamination of the previous active ingredients. A new RP-HPLC method is evaluated for determination of Amoxicillin (AMO) residues in NICOMAC coating machine using Betabasic-C18 (4.6mm x 250mm) 5µm or equivalent, mobile phase of a mixture of 0.05M sodium dihydrogen phosphate: methanol (95:5v/v) adjusted to pH 4.4 with orthophosphoric acid at a flow rate of 1.5mL/min, injection volume 100μL and UV detection at 230nm. The retention time of AMO is 6.292 min and the total run time is 7.0 min. A Linear relationship is obtained in the range 0.03 to 6 ppm with a correlation coefficient of 0.9989, limit of detection 0.05μgmL-1 and limit of quantitation of 0.15μgmL-1. The overall recovery is 100±15%; the relative standard deviation for precision and intraday precision is less than 2.0 %. The validation of the method is performed according to ICH guidelines and USP requirements for new methods, which include accuracy, precision, specificity, LOD, LOQ, robustness, ruggedness, linearity and range.
Keywords: amoxicillin, cleaning validation, RP- HPLC, stability indicating method, NICOMAC coating machine, ICH
AMO, amoxicillin trihydrate; DIW, deionized water; HDPE, High density polyethylene; FDA, Food and drug administration; API, active pharmaceutical ingredient
AMO, is a semi-synthetic penicillin antibacterial drug derived from a fermentation product. Chemically, it is (2S,5R,6R)-6-[[(2R)-2-Amino-2-(4-hydroxyphenyl) acetyl] amino]-3,3-dimethyl-7-oxo-4- thia-1-azabicyclo [3.2.0] heptane-2-carboxylic acid trihydrate having a molecular formula of C16H19N3O5S.3H2O and its molecular weight is 419.4. AMO is a crystalline powder with white or almost white appearance. It is slightly soluble in water, very slightly soluble in ethanol (96%), practically insoluble in fatty oils. It dissolves in dilute acids and dilute solutions of alkali hydroxides.
It may be represented by the structural formula1 as illustrated in (Figure 1). Amoxicillin is stable in the presence of gastric acid and is rapidly absorbed after oral administration. The effect of food on its absorption from tablets and suspension has been partially investigated. The 400 and 875 mg formulations have been studied only when administered at the start of a light meal. However, food effect studies have not been performed with the 200 and 500mg formulations. Amoxicillin diffuses readily into most body tissues and fluids, except for brain and spinal fluid, especially when meninges are inflamed. Its half-life is 61.3 minutes. Most of the Amoxicillin is excreted unchanged in the urine; its excretion can be delayed by concurrent administration of probenecid. In blood serum, amoxicillin is approximately 20% protein-bound. It is similar to ampicillin in its bactericidal action against susceptible organisms during the stage of active multiplication. It acts through the inhibition of biosynthesis of cell wall mucopeptide.2
AMO is official in British Pharmacopeia (BP),1 European Pharmacopeia (EP)3 and United States Pharmacopeia (USP) 4, they include HPLC method for its determination. It is still a limited number of analytical methods that are reported for the determination of AMO including kinetics degradation,5–7 spectrophotometric,8–13 UHPLC, UPLC and mass spectrometry,14–19 thin layer chromatography (TLC),20–22 capillary electrophoresis,23–26 high performance liquid chromatography (HPLC),27–31 in vitro dissolution studies,32–36 amoxicillin residues in animal tissues using SPE-LC,37 SPE-cation exchange,38 in eggs using HPLC-FLD,39 or HPLC-MS40 and in commercial meat and milk samples41 using HPLC-FLD.
According to the best of our knowledge there is no validated method for the determination of amoxicillin residues and application to cleaning machine in pharmaceutical industries. The present work describes the development and validation of an accurate and reliable RP-HPLC method for the determination of AMO residues and application to NICOMAC coating machine.
Materials and reagents
Pure samples: Standard sample of AMO is kindly supplied by Hikma pharmaceutical industries company, Beni-Suef, Egypt with claimed purity of 98.2%.
Chemicals: Methanol HPLC-grade, sodium dihydrogen phosphate dihydrate and orthophosphoric acid are provided from (Scharlau, Spain).
Solvent preparation: Collect a sufficient quantity of fresh deionized water from the DIW loop in a suitable and clean container. Allow cooling to room temperature before use.
Solutions
Standard stock solutions of AMO (1000μg/mL): Weigh about the equivalent to 50mg of AMO from AMO (as trihydrate) working standard. Transfer completely to a 50-mL volumetric flask with aid of 35mL of deionized water. Shake for about 5 minutes, then complete to volume with deionized water.
Working standard solutions of AMO (1.0μg/mL): Accurately transfer 1.0mL from the stock standard solution of AMO into 1000mL volumetric flask, add diluent and sonicate to dissolve. Makeup to the mark with the same diluent and mix well. Inject into the chromatographic system. The chromatogram obtained is shown in (Figure 2).
Instruments and chromatographic system: HPLC system (Agilent 1260 Infinity, Germany) instrument is equipped with an Agilent 1260 Infinity preparative pump (G1361A), Agilent 1260 Infinity DAD detector VL (G131SD), Agilent 1260 Infinity Thermostated column compartment (G1316A) and Agilent 1260 Infinity preparative Autosampler (G2260A) was performed using Betabasic - 4.6-mmx25-cm; 5μm packing L1. Adjust the flow rate to 1.5mL/min using UV Detector adjusted at 230nm with column temperature 8ºC and injection volume of 100μL.
Mobile phase preparation: Dissolve about 10.1g of dihydrogen sodium phosphate dihydrate in 900mL of deionized water. Adjust the pH to 4.4±0.1 with H3PO4. Complete the volume to 1000mL using deionized water. Mix 950mL of dihydrogen sodium phosphate buffer with 50mL of methanol. Filter through a 0.45µm membrane filter.
Construction of standardization curves: Various aliquots of AMO in the range 0.03–6.0µg/mL, are independently transferred from their particular stock standard solutions into separate series of 100mL volumetric flasks and volume is completed to the mark with the diluent and shaked well. Triplicate 100µL injections are executed for every concentration keeping the flow rate at 1.5mL/min and the UV detection at 230nm. The chromatographic system is accomplished using the technique under chromatographic system. The chromatograms are assigned and area under peaks of AMO are determined and the calibration curves are conducted and the regression equations are processed.
Test preparation: To simulate the manufacturing equipment, SS-316 plate (5.08 x 5.08cm2 area) is cut from the SS–316 sheets and is used for all recovery studies. These studies are performed on the SS-316 plate (5.08x5.08cm2 area) by applying solutions of different concentrations (equivalent to 0.1µg/mL, 1.0µg/mL and 10µg/mL) of AMO by using a syringe and drying the plate in air. The plate is swabbed with a swab pre-moistened with methanol vertically and horizontally as shown in (Figure 3). Transfer the cotton swap to HDPE (high density polyethylene) bottle using clean forceps. Add 5mL of deionized water to the bottle. Close with a white cap. Shake the bottle for about 5 minutes. Fill the vial then analyze by HPLC.
Methods development and optimization
System optimization: The selection of mobile phase and the chromatographic system is dedicated to the good separation and resolution, so several conditions have been tested and comprehension different compositions of: (100%) organic solvent, (50:50, v/v); organic solvent: water. Every one of the solvents of the portable stage is filtered through 0.45μ membrane filter paper to expel particulate issue and degassed by sonication, additionally (0.6, 1.2, 1.4 and 1.5mL/min) flow rates were attempted. To get the ideal wavelength of 20µg/mL AMO, the system was optimized within range 200-400nm as showed in (Figure 4). In this manner, 230nm is chosen as the most appropriate absorbance. Preparatory examinations included attempting C18 L1 packing. The best developing system is 0.05M sodium dihydrogen phosphate: methanol (95:5 v/v) adjusted to pH 4.4 with ortho phosphoric acid at a flow rate of 1.5mL/min, injection volume 100μL, the retention time of AMO is 6.292 min and the total run time is 7 min. This selected developing system allows good separation with good Rt values without tailing of the separated bands and good theoretical plates.
Cleaning validation for NICOMAC coating machine: Nowadays pharmaceutical products are manufactured in multi-use facility. FDA protects and promotes public health. Cleaning validation program ensures absence of residues of reaction byproducts and degrades from the previous process/product. The most appropriate cleaning procedure should be developed for the equipment to minimize the cross contamination and there is also necessity to develop and validate the sampling and chosen analytical methods for the compound(s) being cleaned for rinse and swab sampling. Along with taking samples, it is important to perform visual inspection as well to ensure the process acceptability.
Swab sampling: Direct surface sampling can be carried out in several ways, but the most common and widely accepted is swabbing. This involves wiping a predetermined area of the NICOMAC Coating Machine with a swab that has been moistened with a solvent bearing the contaminating compound. Usually the surface is wiped with one side of the swab using a certain number of strokes, then the swap is flipped and the surface is wiped at 90° to the first series of strokes as shown in (Figure 3). Selection of swab is an important part during the cleaning validation program and to maximize the recovery. Two types of swabs are studied–Himedia (having circle head) and Texwipe (having flat head) as shown in (Figure 5). This method of sampling is the most commonly used and involved taking an inert material (eg., cotton wool) at the end of the probe (referred to as swab) and rubbing it methodically across the surface as machine body, hopper, guns, pipes and control panel (Figure 6) The results are represented in Table. 1.
Figure 5 Two types of swabs were studied–Himedia (having circle head) and Texwipe (having flat head).
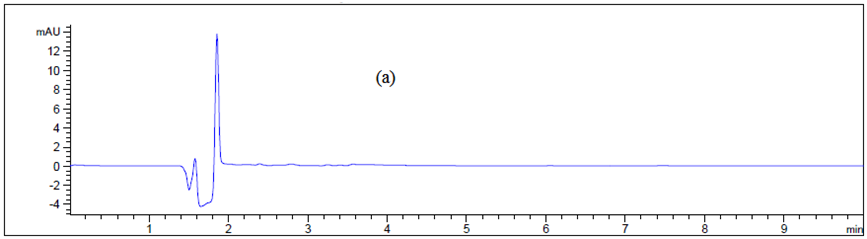
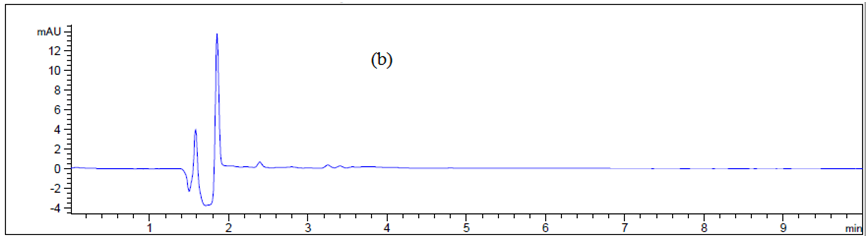
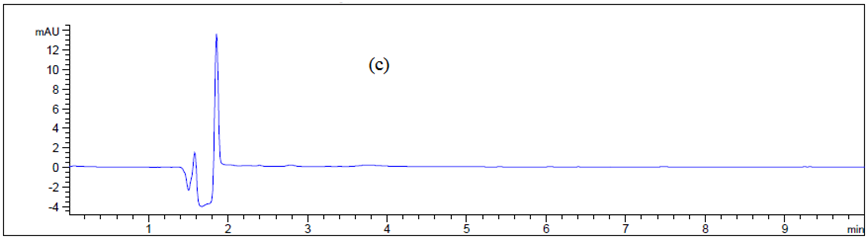
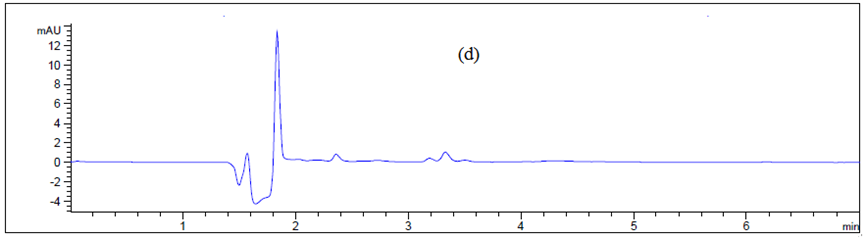
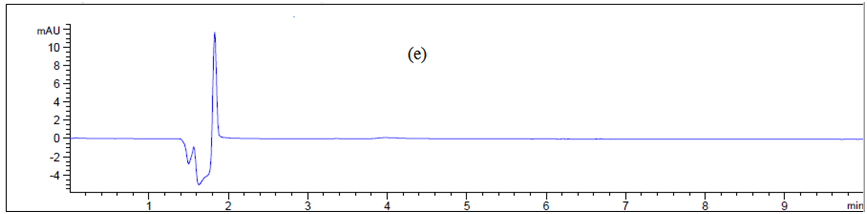

Figure 6 Chromatogram of (a) blank Texwipe swab, (b) Machine body, (c) hopper, (d), guns, (e) pips and (f) control panel.
Rinse sampling: A measured area of a cleaned surface is rinsed or solvent washed and the solvent is collected and tested for traces of contaminants as shown in (Figure 7).
Recently, we have published the validation of Cefaclor.42 The present method was, similarly, validated in accordance with ICH guidelines (ICH Q2R1), for system suitability, precision, accuracy, linearity, specificity, ruggedness, robustness, LOD and LOQ.43
Linearity and range
The linearity of the proposed method is obtained in the concentration range (0.03-6.0μg AMO/mL). Calibration curves are constructed by plotting the obtained peak areas against the corresponding concentrations. The obtained coefficient of regression is 0.9989. Results of linearity are recorded in Table 2.
Serial |
Machine Part |
Location |
Sampling Procedure |
Results |
1 |
Machine Body |
Front side |
Swab |
No Peak Detected |
Top side |
||||
Left side |
||||
Right side |
|
|||
Bottom side |
|
|||
2 |
Mixing Container 1 |
Inside |
||
Outside |
||||
3 |
Mixing Container 2 |
Inside |
||
Outside |
||||
4 |
Air Stirrer (Inside Pan) |
Air stirrer (Inside Pan)1 |
|
|
Air stirrer (Inside Pan)2 |
|
|
||
5 |
Inside Coating Pan |
|
||
6 |
Baffles (inside Pan) |
|
||
7 |
Hopper |
|||
8 |
Guns |
Gun 1 |
||
Gun 2 |
||||
9 |
Pips |
Pipe 1 |
||
Pipe 2 |
||||
Pipe 1 |
||||
10 |
Control Panel |
Top side |
||
Bottom side |
|
|||
Left side |
||||
Right side |
|
|||
Between control |
|
Table 1 NICOMAC coating machine cleaning results by using HPLC
Parameter |
AMO |
Linear |
|
range (ppm) |
0.03-6 |
Slope |
86.6517 |
Intercept |
4.0758 |
Correlation coefficient |
0.9989 |
LOD a (µg/mL) |
0.05 |
LOQ a (µg/mL) |
0.15 |
Repeatability b |
0.101 |
Table 2 Regression and validation parameters of the proposed HPLC method for determination of AMO
aLimit of detection (3.3× σ /Slope) and limit of quantitation (10× σ /Slope).
bRepeatability for n≥5, RSD ≤2.
Precision
Six injections from the working standard solution of AMO 1.0μg/mL was successfully performed, where the RSD below 2.0% as mentioned in the below Table 2.
LOD and LOQ
Many methods are reported to verify LOD and LOQ but the important one is by using calibration curve and regression equation. Diluted standards of AMO solution of 0.05μg/mL, 0.1μg/mL and 0.2μg/mL are prepared for verification of the detection and quantification of the method. Each diluted standard solution is measured in triplicates as shown in (Figure 8).The residual standard deviation of a regression line or the standard deviation of y-intercepts of regression lines may be used as the standard deviation. LOD =3.3×σ /slope and LOQ=10×σ/slope, where σ=the standard deviation of the response as illustrated in Table 2.
Accuracy and recovery
Accuracy is studied by comparing the area of spiked solutions of 0.1, 1.0, and 10µg AMO/mL in solvent with the area of standard AMO in the range of 0.1, 1.0, and 10µg/mL. These data are presented in Table 3. The percent recovery is found to be in the range of 85% to 105%.
Level (ppm) |
Preparation |
Amoxicillin (%) |
Recovery |
||
0.1 |
1 |
88.01 |
2 |
86.7 |
|
3 |
85.25 |
|
Mean |
86.65 |
|
1 |
1 |
100.85 |
2 |
100.72 |
|
3 |
100.63 |
|
Mean |
100.73 |
|
5 |
1 |
102.48 |
2 |
102.47 |
|
3 |
102.47 |
|
Mean |
102.47 |
Table 3 Data of accuracy for AMO
Lab variation method
Ruggedness of the method indicates that the method remains unaffected by small variation in the method parameters as change from day to day, analyst to analyst and different codes in HPLC apparatus, thus the collected data are recorded in codes Table 4.
Parameter(%RSD) |
AMO |
Intraday |
0.118 |
Interday |
0.998 |
Analyst to analyst |
0.114 |
Column to column |
0.869 |
Table 4 Ruggedness of the method
Robustness
Robustness indicates that the changes occurred to the method within the same laboratory. However, robustness can also be characterized as the probability to supersede the analytical method in different laboratories or under different conditions without the status of unusual differences in the obtained results as mentioned in Table 5.
Parameter(%RSD) |
AMO |
Flow rate change (±0.1mL/min) |
1.770 |
pH change of mobile phase (±0.2) |
1.391 |
Wave length change (230±1.0nm) |
0.332 |
Column temperature change(30º±5C) |
0.652 |
Table 5 Robustness of the method
System suitability
The system of the method can be achieved by verification of different factors as injection precision, resolution, theoretical plate and tailing factor, the results and calculated data are presented in the below Table 6.
Item |
Obtained value |
Reference values |
AMO |
||
Tailing factor |
1.07 |
T ≤ 2 |
Selectivity |
4 |
k’ > 2 |
Injection precision |
0.103 |
RSD ≤1% |
Retention time (Rt) |
0.04 |
RSD ≤1% |
Number of theoretical plates(N) |
10366.59 |
N > 2000 |
Table 6 System suitability testing parameters of the developed method
Analyte stability
Analyte stability is used to measure and verify that the solution remains stable under different storage conditions as room temperature against fridge and fresh sample, the obtained results are recorded in the below Table 7.
Condition |
AMO |
Fridge (2-8°C) |
98.22% |
Room temperature (25°C) |
97.10% |
Table 7 Result of stability of analytical solution
Selectivity
An analytical method is considered selective if its calculated data are not changed by other sample components to any significant extent. Compounds, other than analyte, which participate in the analytical signal, are called interfering compounds or interferents. The calculated data are mentioned in the Table 8.
Name |
Amoxicillin |
|||
Effect |
Observed tR |
Peak area |
Degradation % |
|
Test |
Without effect (control) |
6.292 |
89.83479 |
- |
Oxidation effect |
6.254 |
79.9876 |
10.96 |
|
Alkali effect |
6.266 |
74.6756 |
16.87 |
|
Acid effect |
6.281 |
85.2454 |
5.10 |
|
Light effect (Sun light) |
6.289 |
86.2132 |
4.03 |
|
Heat effect |
6.283 |
87.8778 |
2.17 |
|
Placebo |
No peak observed |
No peak observed |
- |
|
Table 8 Results of analysis of forced degradation study samples using proposed method, indicating percentage degradation of AMO
Light degeneration: Accurately weigh 50mg of AMO standard powder previously kept under sunlight for 48 hours and transfer to 50-mL volumetric flask. Add 35mL of the solvent, sonicate to dissolve. Accurately transfer 1.0mL from this stock standard solution of AMO to 1000mL volumetric flask, add diluent and sonicate to dissolve. Shake well and filter, then inject the vials into the HPLC system.
Heat treatment: Retain suitable quantity of AMO working standard in dry oven below 100ºC for six hrs. until all moisture has been driven off and the weight is constant. After cooling to room temperature in a desiccator, accurately weigh 50mg of this powder and transfer to 50-mL volumetric flask. Add 35mL of solvent, sonicate to dissolve and complete to the mark with solvent. Accurately transfer an aliquot of 1.0mL this of AMO into 1000mL volumetric flask, add diluent and sonicate to dissolve. Make up to the mark with the same diluent and mix well. Filter as usual, furthermore inject the vials into HPLC system.
Acid treatment: Accurately transfer 1.0mL from the standard stock solution of AMO into 1000mL volumetric flask, add 100mL of 0.1M HCl furthermore store the acidified solution at warm place for one day. Makeup to the mark. Shake well and filter, then inject the vials into HPLC system.
Alkaline treatment: Accurately transfer 1.0mL from the standard stock solution of AMO into 1000mL volumetric flask, add 100mL of 0.1M sodium hydroxide then keep the basic solution at room temperature for 24 hr. Shake well and filter, then inject the vials into the HPLC system.
H2O2 degradation: Accurately transfer 1.0mL from the standard stock solution of AMO into 1000mL volumetric flask, add 75mL of 3.0% H2O2 then keep at room temperature for two days. Shake well and filter, then inject the vials into the HPLC system.
The proposed RP-HPLC method for the determination of AMO residues in NICOMAC coating machine is precise, specific, accurate and simple and may be successfully applied to quality control analyses during cleaning validation activity as well as routine cleaning programs. Swab recovery study is successfully developed and satisfactory results have been obtained. The results of forced degradation undertaken according to the (ICH) guidelines revealed that the method is selective and can be used for regular routine analysis and stability studies.
Funding
No funding.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
The authors are thankful to the Publishers of the Journal of Analytical & Pharmaceutical Research, MedCrave Group for the publication gift provided.
All authors declare that they have no conflict of interest.

©2018 Hassouna, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.