Journal of
eISSN: 2473-0831


Research Article Volume 5 Issue 4
Correspondence: Mahendra Singh, Department of Pharmaceutical Sciences, Babasaheb Bhimrao Ambedkar University, Vidya Vihar, Rae Bareli Road, Lucknow 226025, India
Received: March 31, 2017 | Published: July 12, 2017
Citation: Raj V, Rai A, Singh M (2017) Detection of Two Bioflavonoids from Methanol Bark Extracts of Acacia Catechu Willd through HPTLC and their Role as Antidiarrhoeal Agents. J Anal Pharm Res 5(4): 00146. DOI: 10.15406/japlr.2017.05.00146
A variety of healing properties have been attributed to the extract of Acacia catechuWilld of family Fabaceae in folk medicine. It has been used for the treatment of dysentery, diarrhoea, colitis, ulcers, piles, boils, skin eruptions, inflammation, psoriasis, leprosy, anaemia and leucoderma. Objective of the study was to evaluate the antidiarrhoeal activity of methanol bark extract of Acacia catechuwilld against experimentally induced diarrhoea by castor oil in albino rats. The methanol bark extract of Acacia catechu(200 and 250mg/kg body weight) was administered orally to two groups of rats (six animals per group) in order to evaluate the activity of the extract against castor oil induced diarrhoea in animal model rats. Two other groups received distilled water (3ml/kg, p.o.) and Loperamide (3mg/kg, p.o.) as suspension (1% CMC) which served as positive control. The effect of the bark extract on castor oil induced diarrhoea and gastrointestinal transit (charcoal meal test) was assessed respectively. In this study, the methanol bark extract was qualitatively assayed for presence of carbohydrates, glycosides, proteins, phytosterols, flavonoids, alkaloids and tannins. However, the different compounds like catechin and quercetin were confirmed by HPTLC. At the oral doses of 200 and 250mg/kg body weight, the plant bark extract showed significant (p<0.05) antidiarrhoeal activity compared to the positive control groups in dose-dependent manner. The present study shows that methanol extract of Acacia catechuhas significant antidiarhoeal activity.
Keywords: antidiarrhoeal activity, acacia catechu, castor oil, HPTLC
Diarrhoea is characterized by an increased frequency of bowel movements, liquid in stool and abdominal pain.1 It is a leading cause of malnutrition and deaths among children in the developing countries like such as Latin America, India and Africa, children may experience between 3 and 10 episodes of diarrhoea yearly.2,3 In 2009, diarrhoea was estimated to have caused 1.1million deaths in children aged 5 and 1.5million deaths in children under the age of 5.4 In terms of etiology, diarrhoea is classified into two types one is infectious that may be caused by a virus, parasite or bacterium while other type is non-infectious diarrhoea that can be caused by toxins, chronic diseases or antibiotics.5,63 Therefore, the cure of diarrhoea is expected to reducing the distress and difficulty of frequent bowel mobility as well as the frequency of faeces.
Acacia catechu Willd occurs naturally in mixed deciduous forests and Savannas of lower mountains and hills in India, Myanmar, Nepal, Pakistan, Thailand, Indonesia, Kenya, Mozambique.7 A. catechu Willd also known as Kattha, Khair and Kaath belongs to family Fabaceae. It has been used for the treatment of diarrhoea, dysentery, colitis, piles, ulcers, boils, skin eruptions, psoriasis, inflammation, leprosy, anaemia and leucoderma.8 A. catechuWilld contains tannins namely, catechutannic acid, acacia catechin, catechu red, catechin, epicatechin and flavonoids- quercetin and quercitrin.9,10
In order to search for newer remedy for diarrhoea, this study aimed at the investigation of the antidiarrhoeal activity of the methanol bark extract of A. catechuWilld on rat model(s) induced by castor oil. The methanol bark extract was also studied for their possible chemical constituents present in it via High Performance Thin Layer Chromatography (HPTLC) which may possessed the antidiarrhoeal activity. HPTLC method is the appropriate method for assessment of chemical constituents present in plant materials. Hence an effort was made to assess the presence of catechin and quercetin present in methanol bark extract of A. catechu by HPTLC finger printing method.
Drugs and chemicals
Loperamide was provided as gift sample (Torrent pharmaceuticals, Sikkim, India), Atropine sulphate (Loba chemie, Pvt. Ltd., Mumbai), Activated charcoal and carboxymetyl cellulose sodium (SD fine chem, Mumbai) was purchased.
Apparatus: CAMAG HPTLC system equipped with Linomat V applicator, TLC scanner 3, Reprostar 3 and WIN CATS–4 software was used.
Collection and authentication of plant material
Certified sample of methanolic bark extract of Acacia catechu was purchased from Herbo Nutra, Mohan garden, Uttam Nagar, New Delhi-59.
Phytochemical testing
The phytochemical analysis of the crude methanol bark extract was chemically assayed for presence of carbohydrates, glycosides, proteins, phytosterols, flavonoids, alkaloids, steroids and tannins. Main chemical constituent’s catechin and quercetin were estimated through HPTLC.
High performance thin layer chromatography
Preliminary phytochemical screening of Acacia catechu was done to identify the chemical constituents and HPTLC finger printing performed for standardization of the drug. CAMAG HPTLC system equipped with Linomat V applicator, TLC scanner 3, Reprostar 3 and WIN CATS–4 software was used to analyse the methanolic bark extract of Acacia catechu.
Sample preparation
40 mg of methanol bark extracts of Acacia catechudissolved in 1ml of HPTLC grade methanol was used for sample application on precoated silica gel GF 254 aluminium sheets.
Developing solvent system
A number of solvent systems and their ratio were tried for methanol bark extract of A. catechu, the satisfactory resolution was found in the solvent toluene, ethyl acetate, and formic acid in ratio of 7:3:0.1respectively.
Sample application
The samples (5µL) were spotted in the form of bands of width 6mm using a 100µL Hamilton syringe on precoated silica gel GF254 plates (20cm×10cm ) with the help of Camag Linomat 5 applicator attached to CAMAG HPTLC system, which was programmed through WIN CATS software.
Development of chromatogram
The mobile phase consisted of toluene - ethyl acetate - formic acid (7:3:0.1) and was used for chromatography run. The plates were developed in a solvent system in a (20cm×10cm) twin through glass chamber saturated previously for 30min with the mobile phase.
Detection of spots
The developed plate was dried by hot air to evaporate solvents, then plate was sprayed with anisaldehyde sulphuric acid reagent and dried at 100ºC in hot air oven for 3min. The plate was kept in photo-documentation chamber (CAMAG Reprostar 3) and captured the images under UV light at 254 and 366nm, respectively. The Rf values and finger print data were recorded by WIN CATS software.
Animals and dose schedules
Albino rats of either sex (180-200gm) were procured. They were housed in polypropylene cages in the animal house of BBDNIIT, Lucknow (Regd. No- BBDNIIT/IAEC/09/2012). Animals were maintained under standard conditions for 12h light and dark cycle respectively for 1week. Animals were fed with standard rodent pellet diet and had free access to water.
Castor oil induced diarrhoea model
The anti-diarrhoeal activity was performed according to method reported by Gnansekar and Perianayagam of methanol bark extract of Acacia catechu. Rats of either sex (180-200g) were fasted for 18h before giving castor oil but allowed to have free access to water ad libitum. The animals were divided into four groups of six each. Distilled water (3ml/kg, p.o.) was given to the first group, which served as a control while the second group received loperamide (3mg/kg, p.o.) as suspension (1% CMC). This served as standard treatment. The methanol extract was administered orally at doses of 200 and 250mg/kg as test solution to third group and fourth group respectively. After 60min of treatment, the animals of each group received 1ml of castor oil orally, by gavage and the consistency of faecal material and the frequency of defecation were noted up to 4h in the transparent plastic dishes placed beneath the individual rat cages.11
(1)
Gastrointestinal motility tests (charcoal meal)
Albino rats (180-200g) were randomly divided into four groups of six animals each. The animals were fasted for 18h prior to the experiments and were allowed free access to water. Each animal was administered with 1.0ml of charcoal meal orally (3% deactivated charcoal in Normal saline) and subsequent treatments were as follows; one group was given normal saline (5ml/kg, p.o.). The positive control group received atropine sulphate (0.1mg/kg, i.p.), while the other two groups received doses of the extract at 200 and 250mg/kg body weight doses orally. After 30min the animals were sacrificed by cervical dislocation and the abdomen opened. The small intestine was dissected out from the pylorus to the caecum and the total distance travelled by the charcoal plug along the small intestine was estimated for both the control and treated groups. For each group, the results were expressed as percentage of the distance travelled from the pylorus to the caecum.12
(2)
(3)
Statistical analysis
Data are expressed as mean ±SD. To determine statistical significance, one-way analysis of variance (ANOVA) followed by Bonferroni t-test were used. A P<0.05 was considered to be statistically significant.
Phytochemical testing
Preliminary phytochemical analysis of methanol bark extract of Acacia catechu revealed the presence of carbohydrates, steroids, alkaloids, flavonoids, tannins. The extract lacked presence of proteins, amino acids, glycosides and saponins. The results are presented in Table 1.
|
Chemical classes |
Tests |
Inferences |
|
Carbohydrates |
Molish’s test |
+ |
|
Fehling’s test |
+ |
|
|
Proteins |
Millon’s test |
- |
|
Xanthoproteic test |
- |
|
|
Biuret test |
- |
|
|
Amino Acid |
Millon’s test |
- |
|
Ninhydrine test |
- |
|
|
Steroids |
Libermann Burchard test |
+ |
|
Salkowski Test |
+ |
|
|
Alkaloids |
Mayer’s Test |
+ |
|
Dragendorff’s Test |
+ |
|
|
Hager’s Test |
+ |
|
|
Wagner’s Test |
+ |
|
|
Glycosides |
Keller Killiani Test |
- |
|
Legal Test |
- |
|
|
Saponins |
Froth formation Test |
- |
|
Tannins |
Gelatin Test Present |
+ |
|
Ferric chloride Test |
+ |
|
|
Flavonoids |
Shinoda Test |
+ |
|
Alkaline reagent Test |
+ |
|
|
|
Zinc hydrochloride Test |
+ |
Table 1 Results of Phytochemical Qualitative tests
HPTLC analysis
The HPTLC chromatogram was run for Acacia catechu bark extract along with standard of catechin and quercetin. In this study HPTLC fingerprinting of methanol bark extract of Acacia catechu revealed several peaks and results are mentioned in Table 2. TLC profile under UV 366nm and 254 nm is shown in the Figure 1 and the corresponding HPTLC chromatograms of standard quercetin and catechin are shown in Figure 2 &3 respectively. The methanol bark extract revealing nine peak with Rf values in the range between 0.04 to 0.84 as shown in Table 2 & Figure 4. Peak 2 and 7 as shown in Figure 4, are confirming the presence of catechin and quercetin in methanol bark extract of Acacia catechualong with other unknown components when compared with the standard peak Rf values of catechin and quercetin.
|
Peak |
Rf Value |
Height |
Area |
Assigned Substance |
|
|
1 |
0.12 |
740.78 |
41182.8 |
Unknown |
|
|
2 |
0.28 |
84.65 |
2003.1 |
Catechin |
|
|
3 |
0.34 |
36.56 |
498.65 |
Unknown |
|
|
4 |
0.4 |
34.69 |
559.79 |
Unknown |
|
|
5 |
0.48 |
41.26 |
390.7 |
Unknown |
|
|
6 |
0.6 |
274.5 |
5489.65 |
Unknown |
|
|
7 |
0.64 |
339.45 |
8112.68 |
Quercetin |
|
|
8 |
0.7 |
205.36 |
7082.97 |
Unknown |
|
|
9 |
0.8 |
298.78 |
14352.2 |
Unknown |
|
Table 2 HPTLC profile of methanol bark extract of Acacia catechu
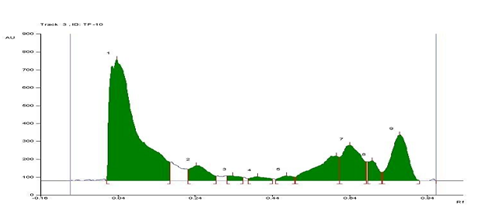

Figure 4 HPTLC chromatogram of methanol bark extract of Acacia catechu showing different peak bands of phytoconstituents.
Castor oil induced diarrhoea
In the castor oil-induced diarrhoea experiment, the bark extract of A. catechushowed a significant increase (using one way-ANOVA, P<0.05) in onset time of diarrhoeal episode, reduction in total number of faeces, total no. of wet faeces with respect to control, as shown in Table 3.
|
Group |
Treatment |
Onset Time of Diarrhoeal Faeces (min) |
Total no. of Faeces in 4h |
Total no. of Wet Faeces in 4h |
Percentage Reduction in Diarrhoea |
|
I (Control) |
Distilled water (3ml/kg, p.o.) + Castor oil (1ml) |
50.0±3.21 |
10.0±0.70 |
8.66±0.88 |
- |
|
II (standard) |
Loperamide (3mg/kg, p.o.) + Castor oil (1ml) |
185.4±4.53 |
3.33±0.33 |
2.66±0.33 |
66.7 |
|
III (treated) |
Extract (200mg/kg, p.o.) + Castor oil (1ml) |
107.0±3.22 |
8.05±0.78 |
7.33±0.66 |
20 |
|
IV (treated) |
Extract (250mg/kg, p.o.) + Castor oil (1ml) |
124.6±2.23 |
6.02±0.57 |
5.63±0.33 |
40 |
Table 3 Effect of Methanol bark Extract of Acacia catechu on wet faeces in 4h, percentage reduction in castor oil induced diarrhoea
Data shown here is in Mean ± SEM and found significant at p<0.05. Six rats were taken in each group (n=6)
Onset time of diarrhoeal faeces, total no. of faeces and total no. of wet faeces were obtained 50.0±3.21min, 10.0±0.70 and 8.66±0.88 for control group in 4hrs respectively. Onset time of diarrhoeal was significantly increased to 107±3.22min and 124.6±2.23min, when treatment group given bark extract at dose 200mg/kg and 250mg/kg respectively. Total number of faeces and wet faeces were significantly reduced to 8.05±0.78, 6.02±0.57 and 7.33±0.66, 5.33±0.33 at the end of 4hrs for treatment group, at doses of 200mg/kg and 250mg/kg bark extract respectively.
The onset time of diarrhoeal faeces was significant increased (using one way-ANOVA, P<0.05) when compared with standard loperamide. Statistical significant differences were observed between control (50±3.21min) vs. loperamide (185.4±4.53min) and control (50.0±3.21min) vs. treatment group (107±3.22min and 124.6±2.23min) at doses of 200 and 250 mg/kg bark extract (using one way-ANOVA, P<0.05) respectively.
The reduction in total no. of faeces in duration of 4hrs was observed when compare with standard loperamide. Statistical significant differences were observed between control (10.0±0.70) vs. loperamide (3.33±0.33) and control (10.0±0.70) vs. treatment group (8.05±0.78 and 6.02±0.57) at doses of 200 and 250mg/kg bark extract (using one way-ANOVA, P<0.05) respectively.
The total no. of wet faeces reduced in duration of 4hrs when compared with standard loperamide. Statistically significant differences (using one way-ANOVA, P<0.05) were observed between control (8.66±0.88) vs. loperamide (2.66±0.33) and control (8.66±0.88) vs. treatment group (7.33±0.66 and 5.33±0.33) at doses of 200 and 250mg/kg bark extract respectively.
There was a significant reduction in percentage of diarrhoeal droppings with respect to loperamide. The reduction in diarrhoea occurrence were observed for treatment group 20.0 and 40.0% at dose of 200 and 250mg/kg respectively. The reduction in percentage of diarrhoeal droppings of both cases was comparable with standard loperamide (66.70%).
Gastrointestinal motility test
Methanol extracts of A. catechushowed significant decrease in mean distance travelled by charcoal meal (cm) with respect to untreated group as shown in Table 4. The mean distance travelled by charcoal meal was 37.10±1.83 for untreated group whereas it was significantly reduced for bark extract treated groups (33.56±0.61, 200mg/kg and 29.23±0.49, 250mg/kg) respectively. The reduction in mean distance travelled by charcoal meal (cm) was found significant comparable with standard atropine sulphate (15.30±0.58). Statistical significant differences were observed between control vs. atropine sulphate and control vs. 250mg/kg dose of extract, respectively (using one way-ANOVA, P<0.05) as shown in Table 4.
|
Group |
Treatment |
Mean Intestinal Length ±SEM(cm) |
Mean Distance Travelled by Charcoal Meal (cm) |
Mean Percentage Movement of Charcoal Meal |
Percentage Inhibition (%) of GI Motility |
|
|
I (Control) |
Normal saline (5 ml/kg, p.o.) + Charcoal meal |
40.83±0.72 |
37.1±1.83 |
90.86 |
-- |
|
|
II (standard) |
Atropine sulphate (0.1 mg/kg, i.p.) + Charcoal meal |
42.5±1.32 |
15.3±0.58 |
36 |
64 |
|
|
III (treated) |
Extract (200 mg/kg, p.o.) + Charcoal meal + Castor oil (1 ml) |
47.93±3.38 |
33.56±0.61 |
70.01 |
29.98 |
|
|
IV (treated) |
Extract (250 mg/kg, p.o.) + Charcoal meal + Castor oil (1 ml) |
47.7±3.06 |
29.23±0.49 |
61.27 |
38.72 |
|
Table 4 Effect of Methanol Bark Extract of A. Catechu on Mean Distance travelled by Charcoal Meal, Percentage of Inhibition (%) using Gastrointestinal Motility Test
Data shown here is in Mean ± SEM and found significant at p<0.05.Six rats were taken in each group (n=6)
Similarly, there was a significant (p<0.05) percentage inhibition in gastrointestinal motility observed for atropine sulphate (64.00%) treated group in comparative to bark extract treated groups. The inhibition in gastrointestinal motility was observed 38.72% for 250mg/kg whereas it was 29.98% for 200mg/kg dose as shown in Table 4.
Around 80% of the world’s inhabitants uses herbal based medicinal plants to cure immediately health problems, which is articulate the significance of multidisciplinary investigation of our natural herbal sources. The study of herbal medicinal plants with the purpose to provide pharmacological indication that may elucidate its therapeutic use. There are a number of in vitro animal models such as isolated ileum of guinea pig, isolated ileum and duodenum of rat or rabbit and measurements of in vivo inhibition of intestinal motility using charcoal meal, Castor oil induced diarrhoea, prostaglandins E2 (PGE2) induced diarrhoea, magnesium sulphate (MgSO4) induced diarrhoea and Enteropooling models.
In this study, the phytochemical analysis of methanol bark extract of A. catechu willd showed the presence of carbohydrates, steroids, alkaloids, flavonoids, and tannins. HPTLC method is also confirming the presence of active constituent’s catechin and quercetin in methanol bark extract.
Earlier studies have shown that the anti-dysenteric and anti-diarrhoeal of various medicinal plants were due to tannins, alkaloids, saponins, flavonoids, steroids, terpenes and glycosides contained in them.13−16
The presence of steroids, alkaloids, flavonoids and tannins in bark extract of A. catechu may be responsible for its antidiarrhoeal effect. The methanol bark extract of A. catechu exhibited significant antidiarrhoeal activity against GI motility. Studies show that activated charcoal sufficiently adsorbs drugs and chemicals components on the surface of charcoal meal particles thereby preventing absorption.17 Hence, GI motility test with activated charcoal was carried out to come across the effect of the methanol bark extract of A. catechu on peristalsis movement. The result also shows that the methanol barks extract suppressed the propulsion of charcoal meal thereby increased the absorption of water and electrolytes.
According to WHO, in diarrhoea three or more loose or liquid stool per day are considered to be normal. It is generally an indication of GI infection, which can be caused by a variety of viral and bacterial organisms, or infection due to contaminated food or drinking-water, or it may vary from person to person as a result of poor hygiene.
Diarrhoea also results from an imbalance between the absorption and secretion in the intestinal tract which results in an extra loss of liquid in the faeces.18 Some diarrhoea cases due to the secretory components while other diarrhoeas may be due to hypermotility. Castor oil induced diarrhoea model is used in this study because autocoids and prostaglandins are involved and these are responsible for diarrhoea in human beings.19 It has been reported that the liberation of ricinoleic acid from castor oil causes the irritation and inflammation in the intestinal mucosa, which leading to release of prostaglandins, which stimulate motility and secretion.20−23
Secretory diarrhoea can be treated by oral rehydration solution (ORS). ORS can reduce the levels of mortality in paediatric and elderly via dehydration but not morbidness. Depends of severity of diarrhoea, in various cases ORS is not adequate hence there is some other additional therapeutics should be used to treat diarrhoea such as antibiotics, spasmolytics, antiprotozoal drugs etc.
To treat the secretory diarrhoea there are some therapeutic agents such as codeine, loperamide, diphenoxylate, lidamidine, bismuth subsalicylate, racecadotril and clonidine which act by decreasing the intestinal movement and are able to stimulates absorption, diminish secretion of water and electrolytes in GI tract, to reduce propulsion, contact time of intestinal content increase with mucosal surface, which favours the absorption.24,25
Castor oil induced diarrhoea is a type of secretary diarrhoea because ricinolic acid, the active component of castor oil, induces diarrhoea by a hypersecretory response. Since the methanol bark extract Acacia catechu effectively inhibited castor oil induced diarrhoea, so it can be supposed that the antidiarrhoeal action was mediated by an antisecretary mechanism. This was also obvious from the decrease of total number of wet faeces in test group in the experiment.
The results of this study showed that the bark extract of Acacia catechu produced statistically significant effect against castor oil induced diarrhoea and is found to be comparable to loperamide (a drug widely employed against diarrhoea disorders which effectively antagonizes diarrhoea induced by castor oil, prostaglandin and cholera toxin).26
Some studies have been carry out in order to discover antisecretory compounds from several herbal medicinal plants used in traditional medicine to cure diarrhoea. To find out the detail results, the extracts of Croton urucurana, C. lechleri, Guazuma ulmifolia and Berberis aristata were studied against intestinal secretion caused by V. cholera toxin. In the case of C. lechleri, B. aristata and G. ulmifolia the isolated compounds were oligomeric proantocyanidins and berberine, respectively. From C. urucurana saponins, steroids, alkaloids, antocianidins and catechins have been isolated. Prontocianidins and catechins probably can be associated with their antisecretory activity.27
In vitro and in-vivo experiments have shown that flavonoids are able to inhibit the intestinal secretory response induced by prostaglandins E2 (PGE2),28 flavonoids have antioxidant properties29 which are presumed to be responsible for the inhibitory effects exerted upon several enzymes including those involved in the arachidonic acid metabolism.30 In this study, presence of quercetin and catechin confirmed by HPTLC which are bioflavonoids, may be responsible for the antidiarrhoeal activity of the methanol bark extract of A. catechu. Presence of catechin (a flavonoids) in methanol bark extract of A. catechu, can also be associated with antisecretory mechanism in diarrhoea treatment.
P.somniferum based herbal formulations as natural remedies are proficient and influential to treat diarrhoea which contains the flavonoids, flavonoids has also been used as complement in treatment of various ailment such as heart diseases, cancer, venous insufficiency, venous ulcers, hemorrhoids and diarrhea.25
It is found that, antidiarrhoeal activity of flavonoids has been ascribed to their ability to inhibit intestinal motility and hydro-electrolytic secretions which are altered in this intestinal condition.31,32 It means methanol bark extract treat diarrhoea in rats, induced by castor oil through inhibiting intestinal motility and hydro-electrolytic secretions, which could be the possible mechanism of action of bark extract.
It has been reported that tannins acts topically as astringent to mucosal surfaces and following oral ingestion it consequently get hydrolyzed and alter the fluidity of the bowel contents (so used in anti-diarrheal remedies).33 Presence of tannins in the bark extract of A. catechu also indicating its astringent property, which may have antidiarrhoeal effect.
The results of this study revealed that Acacia catechu contains pharmacologically active components which have antidiarrheal activity. These attributes may provide the rationale for the use of Acacia catechu in diarrheoa management as a traditional herbal medicine. The results of this study also showed that the bark extract of Acacia catechu produced statistically significant fortification against diarrhoea and was found to be comparable to loperamide. In conclusion, the results of this investigation revealed that methanol bark extract of Acacia catechu contains pharmacologically active components like catechin and quercetin with antidiarrhoeal properties, thus justifying its widespread use by the local population for diarrhoea treatment and other purposes. Inhibiting the intestinal motility and hydro-electrolytic secretions (antisecretory mechanism), which could be the possible mechanism of action of methanol bark extract. Further research is needed to fully investigate the mechanisms involved in the pharmacological activities, to isolate and characterize the active constituents of A. catechu bark. The isolated compound may serve as functional trial products of antidiarrhoeal drugs of natural origin acquiring the required pharmacological activity while lacking untoward side effects.
None.
There was no conflict of interest.
None.

©2017 Raj, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.