Journal of
eISSN: 2473-0831


Review Article Volume 3 Issue 6
Correspondence: Felix Sunday Nworie, Department of Industrial Chemistry, Ebonyi State University, PMB 053 Abakaliki, Ebonyi State, Nigeria
Received: October 31, 2016 | Published: December 15, 2016
Citation: Nworie FS (2016) Bis(Salicylidene)Ethylenediamine(Salen) and Bis(Salicylidene)Ethylenediamine-Metal Complexes: from Structure to Biological Activity. J Anal Pharm Res 3(6): 00076. DOI: 10.15406/japlr.2016.03.00076
Schiff bases and their metal complexes have occupied a central role in the development of co-ordination chemistry as evidenced by the vast number, ease and flexibility of the synthetic procedure, diverse properties, and use as biologically active compounds as antitumor, antibacterial, antifungal and other miscellaneous biological applications. Many reports have demonstrated that Salen and Salen-metal complexes are highly active against several diseases, including cancer. The thrust of this review work is to evaluate the structural nature of Salen and assess the biological applications thereof.
Keywords: schiff base, salen, metal salen, biological applications, structural characterization
C, carbon; N, nitrogen; DMSO, dimethylsulphoxide; PXRD, powder x-ray diffractometer; SOD, superoxide dismutase; Cu, copper; Zn, zinc; Fe, iron; Cr, chromium; Mn, manganese; Co, cobalt; Ni, nickel; Mo, molybdenum; Ru, ruthenium; Rh, rhodium; W, tungsten; Re, rhenium; Os, osmium; Ir, iridium; Pt, platinum; Nd, neodymium; Sm, samarium; Eu, europium; Gd, gadolinium; EXAFS, extended x-ray absorption fine structure; ROS, reactive oxygen species; FTIR, fourier transform infrared spectroscopy; UV, ultraviolet
Schiff bases and schiff base metal complexes
The development of the field of bioinorganic chemistry has increased because the interest in Schiff base has been recognized and many of the Schiff base complexes may serve as models for biological important species.1 Some authors2 noted that in transition metal chemistry, the most commonly used ligands are those that contain O, N donor sets. Schiff bases are characterized by the presence of a C=N double bond (imine) which is bound to an aryl group through the carbon or the nitrogen atom to avoid rapid decomposition or polymerization.
A Schiff base aldimine or azomethine named after Hugo Schiff has a functional group that contains carbon-nitrogen double bond (C=N) synthesized from an aromatic amine and carbonyl compound by nucleophilic addition forming an unstable hemiaminal or carbinolamine followed by dehydration to give an imine as shown in Figure 1.3,4
The formation of a Schiff base by condensation reaction is influenced by pH of the solution, steric and electronic effects of the carbonyl compound and amine. The reaction for the formation in highly acidic solutions is unfavourable as the amine is protonated hindering the nucleophilic ability and in very basic conditions, there is unavailability of sufficient protons to catalyze the elimination of the hemiaminal (carbinolamine) hydroxyl group (dehydration step).5
Consequently, an acid catalyzed condensation reaction of amine with an aldehyde or ketone under refluxing conditions is a very common practice. Generally, aldehydes are used as they react faster than ketones in condensation reactions been that they are less sterically hindered and more electrophilic than ketones which has more electron density. Salen ligand has been noted6 to possess four co-ordinating sites and when co-ordinated to an octahedral metal centre leaves two axial sites where ancillary ligands can co-ordinate. The formation of Salen is shown in Figure 2.
Salen and co-ordination ability
The co-ordination of Salen to metal ions is influenced by the tetradentate property and electronic relevance of the donor atoms. Studies7 shows that it forms planar complexes with various transition metal ions and nitrogen atoms in the ligand have a higher tendency to co-ordinate with metal ions than the oxygen atoms, a consequence of increased basicity of nitrogen atoms over the oxygen atoms. Salen form complexes with metal ions through nitrogen and oxygen donor atoms2 and the metal core can be finely tuned through the appropriate selection of electron - withdrawing or electron donating substituent of different size in the Salen ligands.
The nitrogen and oxygen atoms induce opposite electronic effect, the phenolate oxygen atoms are hard donor and stabilize higher oxidation state of the metal ion while the imine nitrogen atoms are soft donors and stabilize lower oxidation state. Consequently Salen can stabilize many different metals in various oxidation states and as such can be used in different areas.
Faisal et al.,8 observed that the nitrogen basicity is influenced by the electron withdrawing properties of the benzene ring. Consequently, the double bond characters will decrease due to relative small distance that separates the phenol moiety of the molecule and the double bond. The basicity of nitrogen atoms is also influenced by the presence of sp2 hybridization resident in both nitrogen atoms. The double bond decreases the likelihood of flexibility of Salen and makes it less adaptable to co-ordination to metal ions.9
Oxygen atoms in the hydroxyl group are another co-ordination site in Salen and it is expected to be less acidic than the phenolic hydroxyl group. Ortho substitution influences the electron density on the hydroxyl bond making the oxygen in hydroxyl group to be preferred to that in the phenol group. Intrahydrogen bonding in Salen have been shown10 to play a vital role in deciding the co-ordination properties and structure of the molecule. Intrahydrogen bonding occurs between nearby nitrogen atom and hydrogen of hydroxyl group. However, other intrahydrogen pathway may exist. The possibility of two hydroxyl groups to engage in strong hydrogen bonding with the ionization of one of the OH groups exists. Intrahydrogen bonding could occur through atom of ionized oxygen in a hydroxyl group and hydrogen atom of hydroxyl group as shown in Figure 3.8
In Schiff bases, hydrogen bond is exceptionally very strong and Salen ligand is planar with adequate intramolecular distance which favours the formation of intramolecular hydrogen bond.11 Consequently, the electron density on the hydroxyl group is increased by the electron donating groups on the phenolic ring enhancing the strength of H-0 bond and co-ordination of a metal to Salen ligand leads to the shifting of the C=N stretching frequency to a lower frequency showing a decrease in the C=N bond order a consequence of co-ordinate bond formation of the metal with the azomethine nitrogen lone pair.12
Similarly, C-0 stretching frequencies have been reported12 to occur in the region of 1350-1410cm-1 however, C-0 stretching frequency can shift to a higher frequency if oxygen in the phenolic group forms bond with the metal ion.
Low broad intensity band of about 500nm observed in metal complexes is attributed to d-d transitions of the metallic ion. The transition is Laporte forbidden and of low molar absorptivity. The possibility of absence of n- band exists as metal -nitrogen bond stabilizes the electron pair resident on nitrogen atom.13 Ligand to metal charge transfer bands appears as a strong band between 390 and 430nm and has been observed to occur from the orbitals of Schiff bases to the d-orbitals of metals.14
Some authors15,16 noted that bis(salicylidene)ethylenediamine being a tetradentate chelating ligand gives metal complexes of fairly rigid structure. As a consequence, one metal ion co-ordinates through the four co-ordination sites due to the presence of two nitrogen and two oxygen atoms to form three chelate rings. The basicity of both nitrogen atoms which leads to increased stability of the metal complex is a consequence of the double bonds attached to the nitrogen atoms.
Schiff bases derived from substituted salicyaldehyde and alkylamines have been noted2 to exhibit four bands in the near UV which is however solvent dependent. The band region of 240nm was attributed to the n - π transition of the C=N chromophores. The band region of 320nm was attributed to n− transition of C=N, 264nm π− electronic transition of double bond in C= N and aromatic ring, 407 - 406 nm ( n− ) transition between C = N and conjugated benzene ring.
Schiff bases and their metal complexes have occupied a central role in the development of co-ordination chemistry as evidenced by the vast number, ease and flexibility of the synthetic procedure, diverse properties, and use as biologically active compounds.17 Consequently, there is growing interest in the chemistry of Schiff bases and their metal complexes since they offer divers opportunities of fine tuning the metal centered electronic factor, induces substrate chirality and enhances solubility of heterogeneous and homonogenous catalysts.
Of all Schiff base metal complexes, the most Studied are those derived from salicyaldimines as they play significant role in revealing the preferred co-ordination geometries of the complexes. The numbering and naming of salicyladimines complexes of Schiff bases as illustrated18 is shown in Figure 4. Hare18 have shown that most chelates that are formed with transition metal ions are six co-ordinates (Octahedral geometry). Tetrahedral geometry is uncommon in chelates except for divalent metal ions such as Co, Ni, Cu, Zn, Mn, Fe (II) and trivalent Fe.18 The existence of five co-ordinate chelate of salicylaldimine Schiff base metal complexes of Cu (II) and Co (II) have been reported.18 Van Wyk et al.,19 noted that Co (II) complexes from N - aryl salicyladimine ligands have distorted tetrahedral geometries.
Complexes of Salen has been noted20,21 to assume square planar geometry where the metal ion is in the plane formed by the N2O2 donor atoms and solvent molecules or other molecules co-odinates at the two axial positions. In some cases however, the bridge between the imine moieties forces the cis-configuration around the metal centre to a slightly distorted tetrahedral geometry. Cozzi6 has shown that other geometries exist for metal salen complexes where the folding of the six numbered metallocycles and the atoms and centre deviate from the plane defined by the N2O2 donor atoms and may assume an umbrella, stepped or planar molecular conformation as shown in Figure 5.
The co-ordination tendency of N,N1-ethylenebis (salicylidenimine) towards transition metal ions was studied.8 The complexes of nickel (II) and Copper (II) were prepared and characterized using IR and atomic absorption spectroscopy. Results showed co-ordination through the nitrogen atoms in the ligand followed by the attachment of the oxygen atoms. In similarity to this,22 noted that the co-ordination process makes the ligand to accommodate the metal ion so as to satisfy the co-ordination number. Consequently, the co-ordination sites are placed in planar form so as to maintain two monodentate auxiliary ligands that may be available. The study revealed the formation of binuclear copper (II) complex with formula Cu2LCl3 where L= Salen.
Kendre et al.,23 observed that there is an ultraviolet band shift and intensity alternation of ligand in complexation of Salen to metal ion indicating the participation of the ligand in Salen - metal complex formation. The study noted that sharp band observed at 1635cm-1 in the 1R spectrum of Salen may be due to the azomethine linkage which during complexation with metal ions shifted to 1640-1. The high frequencies as observed in Salen-metal complexes were a consequence of the co-ordination of the metal ions to Salen ligand through the azomethine linkage. The study indicated that Salen has a strong band at 3251cm-1 as a result of phenolic hydroxyl group and was absent in metal-Salen complexes. This was attributed to ligand deprotonation and formation of metal - Salen complex through this co-ordination site.
Similarly, studies24 have shown that the IR spectra of Salen ligand in the region (3051- 3059) cm-1 indicates the presence of hydroxyl group a confirmation of the existence of hydrogen bonding in the ligand. Lloret et al.,25 studied the solution chemistry of N, NI- ethylenebis (Salicylideneimine) and its copper (II), nickel (II) and iron (III) complexes by the potentiometric determination of protonation -deprotonation equilibrium of the Schiff base, its organic fragments and the complex formation in dimethylsulphoxide (DMSO) water mixture (80:20 wt/wt) at 25°C and 0.1mol dm-3 potassium tetraoxochlorate (V) (KClO4) ionic strength. The deprotonated ligand Sal2en2- derived from H2sal2en (original ligand) exhibited a planar configuration in the presence of copper (II) and nickel (II) ions.
The study noted that dearth of literature material on the thermodynamic data on the formation of Salen complexes is due to the insolubility of the neutral Schiff base and their metal complexes in water, the hydrolytic decomposition of the Schiff base in aqueous solution to yield their organic starting fragment (aldehyde and primary amine) and if hydrolytic decomposition is not attenable, high acidic media would be required to dissociate the stable polydentate Schiff base metal ion complexes. Iron (III) salen complex FeIII (Salen)Cl is soluble and stable in dimethylsulphoxide (DMSO) - water 80:20wt/wt mixture which however in acid media undergo hydrolytic decomposition. The conductivity measurements of Fe(Salen)Cl solutions indicated a strong 1:1 electrolyte and further proves the presence of the cationic specie Fe Salen]+ in solution.
Spectrophotometrically, (Lloret et al. 1983) noted that complexes of Fe (Salen) Cl2, Cu(Salen) and Ni(Salen) yields dark red, purple and orange coloured solutions respectively in DMSO-water (80:20 wt/wt) mixture. The UV-vis spectra and molar absorptivity of the complexes as measured indicated absorption bands at 485nm (£=3.74 x103) and 316nm (£=1.3 x 104) for Fe (Salen)+, 359nm (£= 9.29 x103) and 568nm (£=298) for Cu(Salen) and 403nm (£=6.18 x 103 Mol-1 dm3 cm-1) for Ni(Salen).
In solution, complexes of the type M(Salen)(n-2)+ are formed but on potentiometric titration with KOH has revealed the formation of hydroxo complex for iron (III) species owing to dimerization of Fe (Salen)+ to Fe (Salen)2O exists whereas no hydroxo complex formation is observed in Cu(II) and Ni(II) Salen complexes.25 The study noted that the stability of (Fe(Salen)]+ is due to co-ordination of phenolate group but for Cu(salen), it is as a result of phenolate and imine co-ordinated groups.
The vibrational spectra of N, N1-ethylene bis(salicylaldiminates) of Ni (II), Cu (II) and Zn (II) have been experimentally and theoretically studied 26. In the work, band assignments and potential energy distribution of normal vibrations in internal co-ordinates and thermodynamic functions of gas phase complexes at the temperature of 298 and 800k were studied. Results indicated that mass spectrometric studies of the superheated vapours of Ni(Salen), Cu(Salen) and Zn(Salen) do not undergo thermal decomposition up to approximately 1000k.
Biological applications of salen complexes
Among metal ions of biological importance, Cu (II) ion involves in a large number of distorted complexes27 and over two decades considerable attention has been paid to metal complexes of Schiff bases containing Nitrogen and other donor atoms.28,29 Bioinorganometallic chemistry studies metallic complexes and their biological applications including the design of new drugs that is more effective than those already known.30
The interaction between native DNA and Iron (III) - N, N1- ethylenebis (salicylideneiminato) - chloride in aqueous solutions using UV-vis, circular dichroism, thermal denaturation and viscosity measurement was studied.31 The study shows that electrostatic binding exists between Fe(Salen)+ cation and phosphate groups of DNA instead of the perceived intercalative grooving.
Zhou et al.,32 synthesized and applied Co (Salen) complex as oxygen activator for the catalytic oxidation of a lignin model polymer using water as solvent, a molecular oxygen and hydrogen peroxide as oxidants. The oxidation effect of Co (salen) was tested using FTIR, 13C-NMR and GC-MS. The study revealed that Co (salen) is important for catalytic oxidation reactions such as CԺ- alcohol oxidation, aromatic ring cleavage, CԺ-Cβ side chain cleavage, β- O-4 cleavage and demethoxylation reactions. Specifically, the catalytic oxidation ability of Co(Salen) was shown by the formation of aldehyde as illustrated by GC-MS result a consequence of β- O-4 bonds oxidative cleavage.
Some authors23 synthesized, characterized and studied the antimicrobial activity of new metal complex of La (III) ion with a tetradentate Schiff base derived from salicylaldehyde and ethylenediamine. The dark yellow electrolytic, diamagnetic and octahedral geometric complex of formula C16H22N2O5Cl3La has maximum absorption at 410nm. The 1:1 metal to ligand mole ratio complex formed was characterized using FTIR, Molar conductivity, magnetic susceptibility, UV-Vis and powder x-ray diffractometer (PXRD). The activity of the ligand and the complex prepared was tested against gram positive E-coli and staphylococcus aureus and gram negative Aspergillus niger and Alternaria. Result revealed that the complex exhibited enhanced activity of inhibition on the growth of the bacteria compared to the ligand. The co-ordination chemistry of manganese and iron has been a subject of much interest especially in their application in biomimetic chemistry, oxidation catalysis and magnetism due to their central position in the periodic table as they can toggle easily between several oxidation states. Manganese (Salen) complexes have been shown to possess superoxide dismutase (SOD) activity that lasts for weeks an indication of excellent SOD mimics.33
Studies33,34 have shown that superoxide dismutases (SOD) catalyze the disproportionation of the superoxide ion into dioxygen and dihydrogen peroxide, (See equation 2.1). The study noted that the activity of Mn - SOD increases during the period of oxidative stress, has longer serum half life compared to Cu and Zn - SODs without inhibition by H202 (hydrogen peroxide). As a consequence, Mn-SODs are noted as potential chemotherapeutic agent for treatment of medical conditions such as tissue cell damage due to effect of dioxygen.
2.1
Dismukes,33 Marnett et al.,34 noted that the primary concern of biomimetic catalysis is to develop systems similar to nature which carries out most oxidation by the use of O2 (Oxygen) as Oxidant, experimentally initiated with an environmentally friendly dihydrogen peroxide which does not require an external reductant. Catalases are enzymes that scavenge dihydrogen peroxide produced in the course of dioxygen reduction to protect cells from oxidative damage.33 Similarly, Mn-Salen complexes synthesized as manganese -N, N1- bis (salicylidene) ethylenediamine chloride have been reported35 to possess superoxide dismutase (SOD) and catalase mimetic properties.
Consequently, it has been noted to act as chemotherapeutic agent in neurological disorders arising from oxidative stress and others such as Parkinson’s disease, stroke, multiple sclerosis and Alzheimer’s disease. Similar studies36,37 noted that the mimetic which are group of manganese - Salen complexes has been subjected to biological examination in association with a U.S.A pharmaceutical company Eukarion Bedford M.A and found to protect cells from diseases arising from oxidative stress such as Parkinson’s diseases, Alzheimer’s disease, stroke, multiple sclerosis, motor neuron disease, ischemia or reperfusion in both heart and kidney tissue and excitotoxic neuronal injury. These studies35‒37 identified the potential of Mn - Salen in arresting cellular damage initiated by oxidative stress (caused by harmful O2- and H2O2) and reactive nitrogen oxides; nitrosative stress (initiated by high level of NO and ONOO-) by the catalytic breakdown of O2-, H2O2, NO and ONOO- to environmentally friendly O2, H2O, NO3- and NO2- and extend life span as proposed.38
Studies35,39 proposed the mechanism for the dismutation of O2- and catalase mimetic action of Mn-salen. The dismutation of O2- involves the reduction of Mn (III) to Mn (II) by O2- which forms O2. The Mn (II) is oxidized to Mn (III) in the presence of O2- giving H2O2.
Similarly,40 reported the beneficial application of Mn - salen complexes as a synthetic superoxide dimutase (SOD) and catalase minetics in models of oxidative stress. The Mn-Salen complexes though not designed to affect the mitochondria, exhibited “mito protective” activity, prevented respiratory chain abnormality caused by ionizing radiation in rat astrocyte cultures and attenuated ischemia reperfusion injury and mitochondrial dysfunction. Similary, Mn - Salen complexes play pharmacokinetics and cytotoxic role in addition to the low molecular weight, antioxidant, superoxide dismutase and catalase and calalytic scavenging mechanism and action against multiple destructive species placing it above other antioxidants.41
Salen manganese complexes have been shown to be beneficial in vivo models for neurodegenerative disorders, radiation injury, endotoxemia, age related impairment and radiation injury to kidney, lung and skin.40,42 As a catalytic mimetic, Mn-salen is oxidized to an oxomanganese salen complex by H2O2 liberating water. Consequently, the Mn-salen complex is reduced by H2O2 to generate the water and oxygen.
Metal -Salen compounds with magnetic properties which can be employed as magnetic materials and magnetic drugs was invented.43 The study revealed that the percentage of metal salen complex compound with a crystal grain size of 1µm or more 3µm or less is preferable and the crystal grain size of 100nm or more to 500nm or less is preferable to ensure passage and retention through the cell capillaries and retain the autoferromagnetism respectively. The study was generalized to Salen - metal complexes such as Fe, Cr, Mn, Co, Ni, Mo, Ru, Rh, Rd, W, Re, Os, Ir, Pt, Nd, Sm, Eu Os, Gd and their derivatives substitution.This work was in support of the previous work by the same authors44 on metal salen complex derivative and process for production thereof where a target component containing an enzyme, an antigen, a peptide, a protein, an antibody or an oligonuclectide and a medical molecule is allowed to bind to the metal - Salen complex through the amide or disulphide bond.
The study revealed that iron-Salen complex has magnetic properties and also antitumor activity and can be guided to the target site whereas the pharmacological effects can be locally concentrated by administering the iron salen complex to an organism (human or animal) and applying magnetic field externally to the human/animal body by allowing the iron Salen complex to bind to a medical molecule. The authors44 claimed that the magnetic medicine containing a metal Salen complex compound can be guided to an affected tissue of a living animal body after systemic application with an external magnetic field. The iron - Salen complex compound produced by crystallizing iron-Salen complex under a rapid cooling condition was claimed to have an antitumor effect and auto -magnetic in nature and does not rely on magnetic substance carriers/external magnetic field. Some workers45 studied the correlation between active centre structure and enhanced dioxygen binding in Co(Salen) nanoparticles and characterized the structure and ligand environment by in situ infrared, Raman, and x - ray absorption spectroscopies. Precipitation with compressed antisolvent technique was used to prepare Co(Salen) nanoparticles from commercially available Co(Salen) referred to as unprocessed exist as dimeric specie of square pyramidal co-ordination geometry with no traceable oxygen (O2) binding activity at room temperature. The increased binding activity of Co(Salen) nanoparticle was attributed to the unusual distorted tetrahedral geometry unlike the commercial unprocessed Co(Salen) with planar co-ordination geometry. Results from X - ray absorption near edge structure (XANES) indicated that there is oxidation of Co (II) to Co (III) as shown in the vibrational data and increase in oxygen co-ordination number in extended X-ray absorption fine structure (EXAFS) upon O2 co-ordinating to Co(Salen) nanoparticles.
Co(Salen) is the first reported synthetically reversible Co(II) oxygen carrier capable of binding dioxygen in the solid state, believed to form O2 adduct consisting of dimeric Co(Salen)2 O2 units and said to be inactive.46 Co(Salen) binds oxygen similarly to iron in the form of ferrous heme in proteins such as myoglobin and hemoglobin.
The oxygen carrying capacity of Salen complexes with Ni (II), Zn (II), Cu (II) and Fe (II) transition metal ion in dimethylsulphoxide (DMSO) was studied.47 The result indicated that the complexes are potential oxygen carriers with the degree of carrying capacity in the order Co (II)> Fe (II)> Cu (II). The study noted that the inability of Ni (II) and Zn (II) Salen complexes to transport dioxygen is due to lack of co-ordination with DMSO. They are unable to absorb oxygen in the form of O2- or O22- and as such inactive. Also,48 investigated the oxygen carrying affinity of cobalt (II) and manganese (II) complexes of H2Salen. The metal complexes were characterized using FTIR, UV-vis, molar conductivity measurement elemental analysis, NMR, TGA and mass spectroscopy. The square planar complexes were implicated as good oxygen carrying species.
Several authors49,50 has reported on the oxygen binding capacity of Co(Salen) and the effect of co-ordinated ligand to the Co(Salen) complexes and its application in O2 separation and storage. The authors noted that the formation of five co-ordinate square pyramidal complexes enhances dioxygen binding.
Studies46 have shown that Co (Salen) decomposes at 498K with final decomposition at 779K corresponding to CoO and Co3O4 as products. The Co(Salen) complex characterized using UV- visible, IR and magnetic susceptibility measurements exhibited high activation energy from various thermodynamic parameter evaluation. Co(Salen) was reported46 to be red brown crystals that darken on exposure to air due to reversible uptake of oxygen by the complex to form the inactive form.
The co-ordination number of Co(Salen) in anaerobic solution may be four, five or six depending on the co-ordinating solvent. In strongly co-ordinating solutions such as pyridine, species like Co(Salen)(PY) and Co(Salen)(PY)2 are formed whereas in weakly co-ordinating solvent like chloroform only Co(Salen) exists. Similarly, complexes of the form 1:1 (Co:O2) or 2:1 (2Co:1) are formed on oxygenation of Co(Salen) to oxygen. The process of dioxygen binding involves the reductive formation of dioxygen adduct where the CO2+ donates electron to the dioxygen forming superoxide (O2-) ligated to the Co3+. The dioxygen is said to add to five coordinate Co(Salen) specie by replacement of one of the coordinated solvent molecule or as the sixth ligand forming a 2:1 complex or 1:1 co-ordinate complex as shown in Figure 6.50
Darensbourg et al.,51 studied the structural characterization of iron (III) Salen complexes possessing appended anionic oxygen donor ligands. The distorted square pyramidal complex, isolated and characterized using X-ray crystal method was air stable, and form a large dark crystals over a short period of time and at temperature of -20°C .
Ansari et al.,(2009) studied the apoptotic and anti-tumor activities of metallo-Salens (Mn (III)-Salen and derivatives) on cultured human cancer and non cancer cells. The result indicated that Mn (III) Salen and derivatives induce fragmentation and nuclear condensation, affect cell viability and induce apoptosis in human cells through mitochondrial pathway. The study also indicated that Mn (III) salen and derivatives showed preferential cytotoxicity (2 to 5 fold) toward malignant breast cells over non malignant breast epithelial cell and this degree of cytotoxicity is similar to cisplatin a known and established antitumor agent. This observation is in line with the observation with Fe (II) Salen and Fe (III) salen derivatives which induces apoptosis in human embroyonic kidney cells and causes in vitro DNA cleavage activities inversely correlated with their apoptoptic activities in cultured human cells respectively.52,53
Also,52,53 synthesized water soluble Fe (III) Salen complex and investigated the biochemical effects on DNA in vitro and on cultured human cells. The study identified that Fe (III) salen produces free radicals in the presence of a reducing agent dithiothreitol (DTT) and induces damage to DNA in vitro. The result showed that Fe (III) salen induces apoptosis in human cells through mitochondrial pathway and damages DNA in vitro as shown in Figure 7. On administration of Fe (III) salen at concentration of 10µm, HEK 293 human cells experienced nuclear fragmentation and condensation changes which are typical of apopotic cell death. Mitochondrial membrane permeability was affected on treatment with IC50 of Fe (III) Salen to 2.µm of HEK 293 human cells and this resulted in translocation of cytochrome C from mitochondria to cytosol.
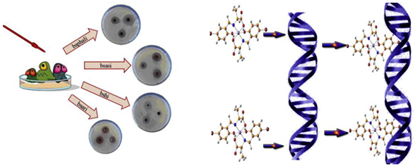
Figure 7 Schematic diagram for antibacterial activity and DNA interaction of metal complexes through intercalative binding.
Similarly,52 studied the relationship between DNA damage potential and biochemical activities using Fe (III) Salen derivatives with varying substituent’s. Result indicated that nature and position of substituents play vital role in determining the apoptotic ability of Fe (III) Salen and that in vitro DNA cleavage activities of Fe (III) Salen complexes are not important for human cell apoptotic activities. In brief, the result showed that Fe (III) Salen induces nuclear fragmentation, activates caspages and apoptosis and affects viability in human cultured cells.
Studies 54 synthesized gold (III) complexes of bis (salicylidene) ethylenediamine characterized it using proton NMR, mass spectrometry, elemental analysis, melting points and infrared spectroscopy and applied it in the cytotoxic study of oesophageal cancer cell lines. The result from in vitro cytotoxic study indicated that gold (III) Salen complex exhibited moderate activity and quenched the proliferation of WHOCI and WHCO6 cancer cell lines with 1C50 range of 19.02-45.27µm and 10.03-68.54µm respectively.
The catalase, dismutase and peroxidase activities of manganese (III) Salen complexes named as Euks, 131, 132, 141 and 142 were evaluated.55 The study noted that the Mn (III) Salen complexes showed exceptional catalase and peroxidase activities whereas the dismutase activity is related to other known compounds. Data from the study revealed that the biological (antioxidant) activities of the complexes are modulated by the position of the substituents on the ligand as well as the central metal manganese atom. Enzymatic antioxidants such as superoxide dismutase, catalase and glutathione peroxidase and non enzymatic antioxidants such as organic molecules like vitamin C and E interact or attack free radicals effectively and destroy or terminate their chain reactions before important molecules in the system are damaged.
This study is in line with the fact that Mn(Salen) complexes with superoxide dismutase and catalase mimicry act as free radical scavengers and has helped in bridging the gap or imbalance between reactive oxygen species (ROS) production and consumption. Mn(Salen) complexes has been noted to be beneficial in handling array of disease states emanating from high level of ROS such as structural and functional injuries and in augmenting immune defense systems.40,56
Uddin et al. 7 studied the complexes of V03+ with dibasic tetradentate Schiff base physicochemically using IR, UV and NMR analysis as well as elemental, magnetic and molar conductance measurements. The electronic data results
indicated - (phenyl ring), n- (HC=N) and charge transfer transitions and based on the data obtained octahedral geometry was proposed for the complexes. The elemental analysis data and molar conductance measurement showed the formation of 1:1(M:L) and non -electrolytic nature of the complexes. The complex synthesized was tested for antibacterial and antifungi activities where the antibacterial activity is effective whereas antifungal was moderate.
Metal complexes as drugs
Metal complexes and ligands show enhanced activity against bacterial agents. Most complexes show enhanced activity over the ligand as explained by chelation theory.58 Chelation makes the complex a more powerful and potent bactericidal agent, thereby killing more of the bacteria than the ligand. This is rationalized in the basis that ligand posseses an azomethine (C=N) bond. Ligands that have hetero donor atoms (N and O) inhibit enzyme activity and enzymes that need these groups for their activity appear to be more susceptible to deactivation by metal ions on co-ordination. Consequently in a complex the positive charge of the metal ion is partially shared with the hetero donor atoms (N and O) resident in the ligand and there may be -electron delocalization over the whole chelating system.59
The type of ligands and the oxidation state of the metal remains a critical factor in the structure of metal ion complexes which regulates the biological activity of the metal based drugs as oxidation state often determines or dictates a particular co-ordination geometry.60
Mechanism or mode of action of metal complexes as antimicrobial agents
Mechanism or mode of action of antimicrobial agents metal complexes have been implicated to involve various targets in an organism such as:
Metals and metal complexes along history have played key role in modern chemotherapy and development of pharmacy.66 The d-block elements and complexes have been shown to have good cytotoxic and photochemical, antimicrobial or both activities.67 Some authors68 studied the spectral, computational, electrochemical and antibacterial properties of Iron (III)- salen complexes. Preliminary antibacterial screening revealed that iron (III) salen complex was effective against chemically important gram negative Escherichia coli (7.2µgmL-1) than Gram positive Staphylococcus aureus (158.3µgmL-1). Similarly,17,69,70 have demonstrated that inorganic metal compounds are used as chemotherapeutic agents and that some drugs have enhanced activity when administrated as metal complexes and not as free organic compounds.
There is growing interest in co-ordination chemistry in bioligand and metal complex synthesis and application in biological field. The design and synthesis of Schiff base ligand and synthesis have attracted serious considerations owing to their applications in medicine, industries and biological areas.49,71
Abou-Melha et al.,72 noted that the co-ordination of a ligand to metal ion synergistically increase the biological activity of the ligand and decrease the cytotoxic effect of ligand and metal ion. In their study, Brown and co-workers73 observed that a compound with long lipophilic and (a long hydrocarbon chain such as propanol) would interact with cellular components and enhance transport of the compound to the active /required site thereby increasing the biological activity.
The biological activity of metal -based drugs is regulated by the type of ligand and oxidation state of the metal and as such is very critical factors in the structure of metal ion complexes. Studies have shown that the co-ordination geometry is determined by the oxidation state of a metal ion and also, the existence of different geometries helps in the manufacture of compounds with stereochemistry that is novel and not obtainable in the group of pure organic molecules.74
Even though metals and metal complexes have played significant role in the development of pharmacy and chemotherapeutic drugs, not much attention have been paid to it and as such remained a tiny minority of all therapeutics in today drug industry.60,75
Hu et al.,76 synthesized, characterized and studied the antimicrobial activity of a hetero trinuclear manganese (III)-iron (II) complex derived from N, N’-bis(5-methylsalicylidene)-1, 2- diaminoethane of formula (Mn(salen) H20)2 (Fe(CN)6) using elemental analysis, IR and single crystal x-ray crystallography. The study shows that the bis-Schiff base ligand ligates to the Mn atom through phenolate O and imine N atoms with each Mn atom in the complex assuming octahedral co-ordination. The study noted that IR spectrum band of the ligand Salen contains medium C-O absorption at 1237cm-1 and this disappeared on complexation with the appearance of new band at 1086cm-1 in the complex spectrum an indication of ligand metal co-ordination through the protonated form. The C=N stretching frequency of the ligand has an absorption band at 162cm-1 which upon co-ordination to the metal leads to a decrease of the absorption band to an intense band at 1615cm-1 an indication of the co-ordination of the imine nitrogen to the metal centre.
The disk diffusion method was used in the antimicrobial activity screening and result indicated that the complexes have more effective activity than the free Salen Schiff base. The observation was explained on the basis of the greater lipophilic nature of the complexes than the ligand a consequence of chelation theory.73
Co-ordination of ligands to metal ions reduces the polarity of metal atoms to a significant extent as a result of the ligand orbital overlap and practical sharing of positive charge of the metal atoms with donor ligand atoms (Salen N202 donor atoms). Consequently, there is delocalization of P-electrons over the whole chelate ring leading to increased metal complex lipophlilicty which in turn enhanced penetration of the complexes into the lipid substrate and blocks metal co-ordination sites on enzymes of micro-organisms.
Studies 53 investigated the biochemical effects of water soluble Fe (III) -Salen complex on DNA in vitro and on cultured human cells. The study indicated that concentration of Fe (III)-Salen as low as 10µm when applied to human cells elicited morphological changes, nuclear condensation and nuclear fragmentation typical features of apoptotic cell death. This was explained on the basis that Fe III (Salen) complex produces free radicals that induces DNA damage. Similarly, the administration of Fe (III) -Salen complex induces apoptosis in human cells through mitochondrial pathway and leads to translocation of cytochrome C from mitochondria to cytosol which affects the permeability of mitochondria membrane.77‒83
In conclusion, the study of Salen and its complexes owing to its ease of manipulation to suit the required design has revolutionalized co-ordination, bioinorganic and other related field of chemistry, physics and biology. There is no doubt that this wonder compound will provide succor to biological areas of interest like cancer, bacteria and fungi (antimicrobial) treatment. It is believed that the future of this area relies on the search for efficient biodegradable drug delivery systems to carry the active compounds, which should increase water solubility and blood circulation life-time, and enhance selectivity towards the needed tissues such as cancer tissue. In Furtherance to this, the use of multifunctional drug delivery carriers would allow the attachment or encapsulation of the Salen compounds, together with imaging agents and or other drugs.
The contributions and necessary advice of colleagues of Industrial Chemistry Department, Ebonyi State University, Abakaliki is highly appreciated.
Author declares there are no conflicts of interest.
None.

©2016 Nworie. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.