Journal of
eISSN: 2473-0831


Research Article Volume 13 Issue 1
1Chromogen, Biotech Park, Salt Lake, India
2RKMV Research Institute, India
3JB Roy State Ayurvedic Medical College & Hospital, India
4RG Kar Medical College & Hospital, India
Correspondence: Dr. Alok K Hazra, CEO, Director, Chromogen, Biotech Park, EN 24, Sector V, Salt Lake, Kolkata, India
Received: January 04, 2024 | Published: February 6, 2024
Citation: Hazra AK, Chakraborty B, Pandit S, et al. A rapid HPTLC fingerprinting technique for identifying the various geo-floral origins of honeys. J Anal Pharm Res. 2024;13(1):5-8. DOI: 10.15406/japlr.2024.13.00432
Honey has several nutritional and therapeutic uses due to the presence of different bioactive compounds.These substances are derived from floral nectar. Therefore, it becomes essential to identify the distinct chemical profiles of honey samples. The aim of this research was to provide an accurate, straightforward, and sensitive HPTLC approach for different types of honey verification. Eight mono floral honeys (mustard, eucalyptus, litchi, orange, tea, Indian plum, black plum and pineapple) were collected from different origin of West Bengal (eastern India) and examined. Standard procedures were followed to check the quality of each honey.The flora was identified by microscopically examining the pollen found in honeys. High-performance thin layer chromatography (HPTLC) was used to analyze lipophilic fractions of each honey. Chromatographic results identified distinct patterns of bands with specific Rf values for each type of mono floral honey. HPTLC is a simple and effective method for routine analysis and verification. It can serve as authentication for different types of honey.
Keywords: honey, honeybee, flora, reducing sugar, pollen, HPTLC
Honey is a sweet, energizing, medicinal and functional food. Honeybees (Apis sp.) make it from the nectar of flowers. It is one of the popular product in the food manufacturing, pharmaceutical and cosmetics industries. More than 300 types of floral honey are available worldwide.1 The chemical composition of honey depends on number of factors, including as its botanical or geographical origin, climatic variations, species of bees, processing methods, and preservating techniques.2 It is essential to maintain nutritional value, authenticity, and purity of honey. The Codex Alimentarius standard, USFDA, Indian and European Directive outline the requirements for its quality.3–6 Determining its moisture content, sugar content, water-soluble solid content, mineral content (ash), acidity, diastase activity and hydroxymethylfurfural (HMF) concentration are necessary for quality assurances. It is a thick viscous liquid with 60–85% carbohydrates (primarily glucose and fructose), 15–22% water, 0.1–0.4% protein, 0.2% minerals (ash), and trace amounts of vitamins, enzymes, amino acids, and other biologically active compounds like flavonoids, phenolics, and essential oils.7 The composition of honey may differ on the sources of nectar. It's multiple health benefits also rely upon the way it's naturally blended. Honey has been employed for thousands of years as an antibacterial, wound therapy, and digestive remedy.8,9 Recent studies have shown that it helps with diabetes, cancer, inflammation, immunomodulation, and cardiovascular diseases (CVDs).10–12
India offers a wide range of honey types, including ripe and unripe, extracted and squeezed, processed and raw, and mono and multi-floral. The quality of Indian honey can vary depending on beekeeping procedures as well as blending, settling, heating, bottling, and storage conditions.13 West Bengal is one of the states in India that produces the most honey with high qiuality. Beekeeping is one of the organized agricultural industries there.14 On the other hand, very little is known about the Indian mono-floral honey profile. The development of honey profiles is mainly based on analyses of the organic phenolic constituents.15 Many different types of analytical techniques have been developed to achieve this. Thus, the main objective of this work was to use high-performance thin-layer chromatography (HPTLC) to differentiate honeys from different botanical mono-floral origins of West Bengal.
Sample collection
Eight (8) mono floral honey samples, namely mustard (Brassica juncea), eucalyptus (Eucalyptus globules), litchi (Litchi chinensis), orange (Citrus sinensis), tea (Camellia sinensis), Indian plum (Ziziphus mauritiana), black pulm (Syzygium cumini) and pineapple (Ananas comosus) were obtained at different botanical sites during various seasons throughout the state of West Bengal (21°25́ to 27°13́ N and 85°50́ to 89°50́ E), India. All unprocessed honey samples were collected freshly in sterile brown containers and stored at 4ºC until analyzed. Before analysis, unwanted items including wax sticks, dead bees, and comb remnants were eliminated from the samples.
Varity of honey
Identifying the type of honey was done using the melissopalynological method.16 The name of the honey variety was taken from the botanical name of the plant or plants, and this microscopic technique (Olympus, Japan) used for the determination of the share of predominant pollen grains in a certain honey.
Quality assurance study
Following the methods described in the Indian Ayurvedic Pharmacopeia (2008), the quality of honey samples was determined.17 Specific gravity, loss on drying, acidity, reducing sugar, sucrose, fructose-glucose ratio, commercial sugar, and total ash content were estimated for basic quality assurance investigation.
High performance thin layer chromatography (HPTLC) analysis
The lipophilic fractions of each honey samples were extracted by pure methanol (liquid phase separation). Briefly, 1 g honey was thoroughly mixed in 10 ml methanol and kept overnight (10-12 h) at room temperature (20-22⁰C). In the following day, solvent was completely removed by passing nitrogen gas. Finally, the samples were eluted with 1 ml methanol and filtered (0.5µ nylon filter) using syringe. The test samples were spotted (0.2µl, brand length 8mm, 10 mm gap) with automated Camag microlitre syringe and Camag Linomat applicator on a pre-coated silica gel 60 F254 (Merck KGaA;1.05554.0007) plates (10×10 cm).18 The plates were developed in 10 ml solvent mixture (toluene : ethylacetate : formic acid = 4.5:3:0.2 v/v). The peaks regions in the TLC plate were finally scanned with CAMAG TLC Scanner 3 at 254 nm (D2 lamp) using absorbance mode.19 WinCAT's planar chromatography manager software was used to calculate the Rf values for the bands in the images of the HPTLC plates.
Statistical analysis: Categorical variables were presented as percentages wherever applicable. All data were demonstrated as mean and standard deviation (SD). The statistical analysis was performed using statistical software SPSS v20 (IBM, Chicago, USA).
In the present study, extracts from several mono-floral Indian honeys—mustard, eucalyptus, litchi, orange, tea, Indian plum, black pulm, and pineapple—were analyzed using the HPTLC method. These investigations were conducted with the knowledge that the various classes of chemical compounds found in the lipophilic fractions of honeys might be helpful to determine the botanical and geographic origins of honey as well as its intrinsic value. Furthermore, it could undoubtedly be employed as a fast-screening technique for analyzing honey samples, and its fingerprint will be beneficial for identifying instances of adulteration in particular honey.20 The pollen analysis (melissopalynological investigations) used in this study revealed the existence of specific mono flora (Figure 1). This investigation depended on additional analysis and validated the mono floral honey from each other.16 Furthermore, each honey was checked to assure its quality according to the guidelines of the Indian Ayurvedic Pharmacopeia (Table 1). Mustard honey had a minimum specific gravity of 1.35 and eucalyptus honey a maximum of 1.66. Indian honey should have a specific gravity of 1.35 and a moisture content of no more than 25%, according to Indian Pharmacopeia.17 In this instance, the specific gravity and moisture content limits of each honey sample were met. Since the high sugar concentration in honey tends to disguise the acidity in its taste, it is unexpectedly acidic. In this study, the black plum honey was found to have the highest acidity.

Figure 1 Microscopic identification of pollen in honey of different mono-floral origin. 1= mustard; 2= orange; 3= litchi; 4= tea.
|
Honey varieties/ botanical origin |
Specific gravity |
Loss on drying (%) |
Total ash (%) |
Acidity (%) |
Reducing sugar (%) |
Sucrose (%) |
Fructose- glucose ratio (%) |
Aniline chloride test |
Fieche’s test |
|
Mustard(Brassica juncea) |
1.35 |
24.92 |
0.05 |
0.09 |
61.8 |
1.18 |
8.23 |
Negative |
Negative |
|
Eucalyptus(Eucalyptus globules) |
1.66 |
15.47 |
0.13 |
0.11 |
64.02 |
2.36 |
9.32 |
Negative |
Negative |
|
Litchi(Litchi chinensis) |
1.39 |
17.18 |
0.1 |
0.11 |
63.35 |
1.92 |
5.22 |
Negative |
Negative |
|
Orange(Citrus sinensis) |
1.47 |
15.13 |
0.5 |
0.08 |
64.49 |
2.72 |
8.41 |
Negative |
Negative |
|
Tea(Camellia sinensis) |
1.39 |
17.91 |
0.24 |
0.03 |
66.05 |
2.06 |
6.09 |
Negative |
Negative |
|
Indian plum(Ziziphus mauritiana) |
1.42 |
18 |
0.2 |
0.07 |
61.83 |
1.88 |
5.84 |
Negative |
Negative |
|
Black plum(Syzygium cumini) |
1.48 |
14.06 |
0.09 |
0.13 |
64.26 |
1.64 |
8.08 |
Negative |
Negative |
|
Pineapple(Ananas comosus) |
1.53 |
22.11 |
0.04 |
0.05 |
62.31 |
2.55 |
4.65 |
Negative |
Negative |
Table 1 Compositional quality assurance of mono-floral honey of different botanical origins
The suggested reducing sugar level in honey is less than 65%, so all honey samples were within the range. The recommended fructose to glucose ratio is above 1%.7 In accordance with this study, eucalyptus honey had the highest fructose-to-glucose ratio, followed by mustard, orange, black plum, tea, Indian plum, litchi, pineapple, and black plum honeys. Orange honey had the highest natural sugar concentration (2.72%), although it was still below the advised threshold (less than 5%). Honey's overall mineral content (total ash) stayed below the allowed threshold of 0.5%.3 For the detection of adulteration resulting from the addition of external sugar to honey samples, the Aniline Chloride Test and Fieche's Test were performed.17 Since both tests revealed negative results in every examined sample of honey, it was determined that there was no commercial sugar present in the samples. In this work, we established a standardized HPTLC method and presented chemical fingerprints of lipophilic honey fractions from various mono floral sources.20–22 Different honey samples exhibited distinct patterns of bands, indicating differences in the composition of lipophilic chemical compounds (Figure 2A& 2B). Conversely, the patterns found in some honey varieties exhibit remarkable similarity as well as differences of different honeys (Figure 2C).
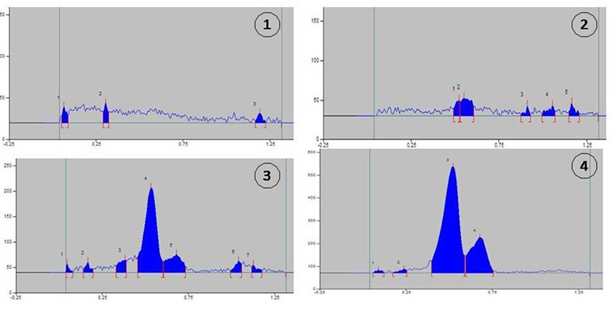
Figure 2a HPTLC chromatogram of honey of different mono-floral origin. 1= mustard;2= Eucalyptus; 3= litchi; 4= orange.
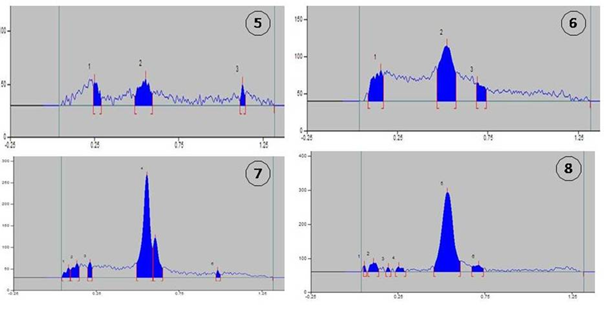
Figure 2b HPTLC chromatogram of honey of different mono-floral origin. 5= tea; 6= Indian plum; 7= black plum; 8= pineapple.
In HPTLC, the retention factor, or Rf value, is significant because it makes it possible to separate and anticipate the constituents from a mixture of molecules.23 Litchi honey showed seven different bands in these HPTLC chromatograms, followed by pineapple six bands, black plum six bands, eucalyptus five bands, orange four, tea three, mustard three, and Indian plum three (Table 2). Therefore, the highest numbers of lipophilic substances were found in litchi honey. Interestingly, almost all honeys (except from mustard) showed a consistent unique band with a maximum area% at Rf=0.53 to 0.56. In eucalyptus honey at Rf=0.55 area percent was 44.38%, in litchi it was 65.35%, in orange 73.26%, in tea 59.32%, in Indian plum 61.96%, in black plum 64.17% and in pineapple 84.19%. At Rf=0.55, the area percent of eucalyptus honey was 44.38%, litchi was 65.35%, orange was 73.26%, tea was 59.32%, Indian plum was 61.96%, black plum was 64.17%, and pineapple was 84.19%. Similarities with litchi honey (16.57%), orange (24.72%), black plum (18.16%), pineapple (5.27%), and Indian plum (11.60%) were also observed, with Rf values ranging from 0.68 to 0.71. One more identical brand was noted at Rf = 1.13: mustard (39.70%), litchi (2.61%), and tea (13.83%). Consequently, distinctive peaks in the HPTLC chromatograms of various honeys distinguished their chemical moieties from one another and created a distinctive signature for mono-floral honeys from various origins. To the best of our knowledge, this is the first study conducted in West Bengal, India, to identify the HPTLC fingerprints of honey provenance. Enhancing quality assurance could be beneficial in getting it accepted by different segments of the population. It's also true that its biological and therapeutic qualities might be reflected in it. To support and validate these results, more research of a similar nature is needed.
|
Honey varieties/ botanical origin |
No. of peaks |
Rf (area %) |
||||||
|
|
|
Rf1 |
Rf2 |
Rf3 |
Rf4 |
Rf5 |
Rf6 |
Rf7 |
|
Mustard(Brassica juncea) |
3 |
0.07 (34.64) |
0.31 (39.66) |
1.3 (39.70) |
|
|
|
|
|
Eucalyptus(Eucalyptus globules) |
5 |
0.53 (16.84) |
0.55 (44.38) |
0.91 (8.26) |
1.06 (16.09) |
1.17 (14.43) |
|
|
|
Litchi(Litchi chinensis) |
7 |
0.05 (1.53) |
0.17 (3.19) |
0.39 (6.25) |
0.54 (65.35) |
0.68 (16.57) |
1.04 (4.51) |
1.13 (2.61) |
|
Orange(Citrus sinensis) |
4 |
0.09 (0.81) |
0.24 (1.21) |
0.53 (73.26) |
0.68 (24.72) |
|
|
|
|
Tea(Camellia sinensis) |
3 |
0.25 (26.85) |
0.56 (59.32) |
1.13 (13.83) |
|
|
|
|
|
Indian plum(Ziziphus mauritiana) |
3 |
0.16 (26.44) |
0.53 (61.96) |
0.69 (11.60) |
|
|
|
|
|
Black plum(Syzygium cumini) |
6 |
0.08 (3.86) |
0.13 (7.35) |
0.21 (4.67) |
0.56 (64.17) |
0.68 (18.16) |
0.98 (1.79) |
|
|
Pineapple(Ananas comosus) |
6 |
0.06 (1.18) |
0.11 (5.72) |
0.20 (1.04) |
0.26 (2.59) |
0.54 (84.19) |
0.71 (5.27) |
|
Table 2 Chromatographic results of mono-floral honey of different botanical origins
The current findings support the importance of the HPTLC technique for assessing samples of Indian honey with varying botanical origins. Their distinct fingerprints are mostly determined by comparing the chromatogram profiles with the observed Rf values. To the best of our knowledge, this is one of the few studies using HPTLC to identify the botanical source of Indian honey samples.
The authors would like to thank Mr. Pankaj Choudhury for providing samples of honey with varying botanical origins.
The authors declare that there is no conflicts of interest.

©2024 Hazra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.