Journal of
eISSN: 2473-0831


Research Article Volume 4 Issue 5
GITAM Institute of Pharmacy, GITAM University, India
Correspondence: Mukthinuthalapati Mathrusri Annapurna, GITAM Institute of Pharmacy, GITAM University, Rushikonda, Visakhapatnam-530045, India
Received: April 26, 2017 | Published: May 5, 2017
Citation: nnapurna MM, Venkatesh B, Krishna CR. Quality by design approach (qbd) for the simultaneous determination of anti–hypertensive drugs (candesartan, irbesartan and hydrochlorothiazide) by rp–hplc. J Anal Pharm Res. 2017;4(5):146-155 DOI: 10.15406/japlr.2017.04.00118
A new reverse–phase liquid chromatographic technique has been developed for the separation and determination of anti–hypertensive drugs (Candesartan, Irbesartan and Hydrochlorothiazide) using QbD (Quality by Design). The present method was optimised by introducing experimental design approach to identify the chromatographic conditions where the adequate separation quality in minimal analysis duration. The relationship among independent variables and critical quality attributes is given by experimental design methodology. The segregation was consummated on Sunfire C18 type column (150x4.6mm; 5µm particle size) as stationary phase; 0.1% acetic acid in water: acetonitrile (33:77% v/v); 0.7351ml/min as flow rate; detection at 225nm. The chromatographic efficiency was investigated for the composite effects of % organic phase and flow and finely optimized by employing central composite design. The method was validated and was found to be accurate, precise and robust. The method was thrivingly exercised with the marketed formulations.
Keywords: hydrochlorothiazide, irbesartan, candesartan, central composite design, rp–hplc, validation
QbD: quality by design; CCD: central composite design; IRB: irbesartan; CST: candesartan; HTZ: hydrochlorothiazide; CAN: acetonitrile; PDA: photo diode array; %RSD: percentage relative standard deviation; ICH: international conference on harmonization
Sartans are used solely or in alliance with other hypertensives during hypertension therapy.1,2 These were also used in treatment of diabetic nephropathy in patients suffering with hypertension with type 2 diabetes mellitus and also in congestive heart failure. These angiotensin II receptor antagonists are given in combinations with hydrochlorothiazide, a diuretic that is very effective in treating blood pressure.3 In modern trends the International Conference on Harmonization (ICH) suggests implementation of Quality by design based experiments in the fields of pharmaceutical product development and also in analytical method development. Optimization of HPLC method very tangled procedure as the separation and other performance criteria depend on various independent variable parameters such as Strength of buffer, mobile phase pH, flow rate, detection wavelength, etc.4 Any significant interaction between those independent variables may lead to the failure of the univariate procedure as the effect of one variable on the response may be in connection with the levels of the other variables involved in the method optimization. Chemometric approach has become a new and better concept for the RP–HPLC method optimization than the traditional approach based on fluky trial and error methodologies as there is reduction in the number of experiments there by lowering reagent consumption. The experimental design methodology explains the concomitance between the sensitivity of the independent variables and chromatographic parameters that critically attributes the method quality.5
Irbesartan (IRB) an angiotensin–II receptor antagonist and also used for the curtailment of renal disease progression in patients with type 2 diabetes.6 It is chemically known as 2– butyl– 3– ({4–.2– (2H– 1, 2, 3, 4– tetrazol– 5– yl) phenyl phenyl} methyl)– 1,3– diazaspiro.4 non– 1– en– 4– one (C25H28N6O) 428.53 g/mol.
Candesartan (CST) is an angiotensin II type 1 receptor antagonist.7 It is chemically tetrazole derivative which is chemically known as – 2– ethoxy– 1– ({4–.2– (2H– 1,2,3,4– tetrazol– 5– yl) phenyl phenyl} methyl)– 1H– 1,3– benzodiazole– 7– carboxylic acid (C24H20N6O3) with molecular weight 440.45 g/mol.
Hydrochlorothiazide (HTZ) is a thiazide diuretic.8 Chemically it is known as 6– chloro –1 ,1–dioxo –3, 4– dihydro –2H –1, 2, 4 –benzothiadiazine –7 –sulfonamide (C7H8ClN3O4S2) with a molecular weight of 297.74 g/mol. The chemical structures of HTZ, IRB and CST were shown in Figure 1A–1C respectively.
Literature survey acknowledge that various methods such as LC–MS.9,10 UPLC.11 HPLC.12–17 Micro emulsion LC.18 HPTLC.19 spectrofluorometric.20 and spectrophotometric methods.21–26 were been developed for simultaneous determination of Irbesartan and Hydrochlorothiazide in biological fluids as well as pharmaceutical formulations.
Similarly, for the simultaneous determination of Candesartan and Hydrochlorothiazide in tablet dosage forms as well as in human plasma few analytical methods such as HPTLC.27 LC–MS/MS.28 UPLC–MS/MS.29 HPLC.30–40 and spectrophotometric.41–44 were developed.
Up to our knowledge till date no method was available for simultaneous determination of these three drugs. Therefore, the main aim of the authors is to develop a chemometric–assisted RP–HPLC method possessing statistically optimized chromatographic parameters with simplest mobile phase and is to validate it as per ICH guidelines.45
Instrumentation
CBM–20A/20 Alite model HPLC system of Shimadzu make, equipped with SPD M20A prominence photodiode array (PDA) detector connected to the system Dell Optiplex 790 loaded with LC Solutions v2.0 is rigged for the integrating and processing of chromatograms.
Materials and reagents
Reference standards of CST, IRB and HTZ (purity >99%) was obtained from Sun Pharmaceutical Industries Ltd., India as gift samples. Acetonitrile, glacial Acetic acid, sodium hydroxide (NaOH), Hydrogen peroxide (H2O2) and hydrochloric acid (HCl) were acquired from Merck (India). All chemicals used were of analytical grade and used as received. The available marketed formulations are IROVEL–H® (Sun Pharmaceutical Industries Ltd., India) with a label claim: 150mg/12.5mg of IRB/HTZ, and CANDESAR–H® (Ranbaxy Laboratories Ltd., India) with a label claim of 16 mg/12.5mg of CST/ HTZ.
0.1% Acetic acid solution (aqueous phase) was made by meticulously transferring 1ml of glacial acetic acid into a 1000ml volumetric flask and make up the volume with HPLC grade water. The climactic solution was sonicated for half an hour and filtered. The stock solutions were prepared by accurately transferring 25mg each of HTZ, IRB and CST in to different 25ml volumetric flasks and all the samples were dissolve using acetonitrile (1000μg/ml) and obligatory supplementary dilutions were fixed from the stock solutions.
Chromatographic conditions
A reverse phase Sunfire C18 type column (150mm length × 4.6mm i.d., 5µm particle size) was used as analytical column for the separation. The analysis was fetched by: Waters make Sunfire C18 type column (150mm×4.6mm i.d., 5µm particle size); 0.1% acetic acid in water and acetonitrile (33:77, v/v) as mobile phase (Isocratic mode); flow of 0.7531ml/min; UV detection at 225nm. All chromatographic conditions were performed at ambient room temperature (25°C±2°C).
Calculations and software
Chromatographic responses were taken by using LC–Solutions v1.25 which is a data acquiring software by Shimadzu. Design Expert 9.0.3 trial version (Stat–Ease Inc., Minneapolis, MN, USA) has been involved for the experimental design and the selection of the runs. The effects of parameters and their statistical interpretation employed in analytical method development were studied and calculated.
Method validation
Linearity: For the linearity studies a consecution of solutions (0.1–200μg/ml) were projected for each drug from their respective stock solutions and 20µl of each solution was injected in to the HPLC system and the respective chromatograms were chronicled. A calibration curve was charted by considering the concentration of the drug solutions on the x–axis and the cognate peak area on the y–axis and the resulting linear regression equation so attained was adapted for the assay evaluation of marketed formulations.
Precision: The method precision was valuated in terms of repeatability. The precision studies were performed by analysing the samples of HTZ, IRB and CST at three distinctive concentration levels i.e., 5, 10 and 20μg/ml for HTZ and 10, 20 and 50μg/ml for IRB and CST. The % RSD of the three assay values (n=3) was calculated. For inter–day precision the study was conducted on three distinctive days i.e. day 1, day 2 and day 3 while the intra–day precision study held on the same day at distinctive intervals of time.
Accuracy: The accuracy of the developed method was appraised in triplet, spiked at three pre–defined concentration levels (80, 100 and 120%) for all three drugs and their percentage recoveries were calculated. The study was checked out in triplet at 9, 10 and 11μg/ml for HTZ and 18, 20 and 22μg/ml for IRB and CST. The percentage recoveries of all drugs were calculated in each case.
Robustness: The robustness for the developed method was performed by varying the chromatographic circumstances which compraise: flow rate (±0.1ml/min), percentage of organic phase in the mobile phase (±2% acetonitrile, v/v) and wavelength (±2nm). Robustness of the method was reviewed in triplet at a concentration level of 10μg/ml of each drug.
Limit of quantification and limit of detection: The limit of quantification (LOQ) and limit of detection (LOD) were based on the standard deviation of the response and the slope of the charted calibration curve (n=3), as described in ICH guidelines Q2 (R1) (45). LOD and LOQ majorly attributes to the sensitivity of the method.
Assay of marketed formulations: Twenty tablets of each brand of IROVEL – H® (label claim: 150mg/12.5mg of IRB/HTZ), and CANDESAR – H® (label claim of 16mg/12.5mg of CST/ HTZ) were procured from the local pharmacy store, weighed and crushed into fine powder. Powder equivalent to 25mg of IRB and CST respectively of each formulation was accurately weighed and transferred into separate 25ml volumetric flasks and made up to volume with mobile phase. The contents of the volumetric flasks were well sonicated about 30min for the absolute dissolution of the entire drugs. The solutions were centrifuged for 15mins in ultra–centrifuge and the supernatant solution was collected and then filtered through 0.45μm membrane which is used as the stock for the formulations. The prerequisite dilutions were further conducted and analysed. The peak areas were recorded from the respective chromatograms.
Optimization of experimental conditions
The main aim of developing the RP–HPLC method is to simultaneously determine CST, IRB and HTZ in bulk and tablet dosage form which are separated from each other with good resolution (RS > 2.0), peak shape.tailing factor (TF≤2) and shorter analysis time (<10 min) which can be achieved by modifying critical HPLC parameters. During the initiatory experimentation, several mobile phase compositions (% organic phase) as well as flow rates were screened to study their influence on the responses. In the optimization phase, several consequential HPLC parameters whose permutation shows an impact on the separation of the three drugs were identified and to determine the optimum combination and the response pattern a face centred central composite design (CCD) with the two independent variables (% organic phase and flow rate) each at three levels were used as shown in Table 1. A CCD–aided response surface methodology (RSM) based design of experiment was inked to acquire ideal combined effect of % organic phase (acetonitrile) and flow rate on the chromatographic responses. The pooled influence of independent variables each at triplet levels on the chromatographic responses were investigated.
|
Variables |
Levels |
||
|
–1 (Low) |
0 (Medium) |
1 (High) |
|
|
Independent |
|||
|
% Organic phase (% ACN) |
77 |
82 |
87 |
|
Flow rate (ml/min) |
0.6 |
0.7 |
0.8 |
|
Dependent |
|||
|
Rs(HTZ−IRB) |
= Resolution of IRB |
||
|
Rs(IRB−CST) |
= Resolution of CST |
||
|
THTZ |
= Tailing factor of HTZ |
||
|
TIRB |
= Tailing factor of IRB |
||
|
TCST |
= Tailing factor of CST |
||
|
Totalanalysistime(min)Total analysis time (min) |
= Total run time |
||
Table 1 Experimental variables and coded levels considered in the Central composite design
From CCD–aided RSM, nine experimental runs were implemented and the influence of the above said variables at 3 level on the chromatographic responses (resolutions tailing factors and total analysis time.retention time of CST) was investigated (Table 2). The resulted chromatograms from the experiment are depicted in Figure 2a–2i.
|
Run |
Independent Variables |
Chromatographic Responses |
||||||
|
% Organic phase |
Flow rate |
Rs(HTZ−IRB) |
Rs(IRB−CST) |
THTZ |
TIRB |
TCST |
Totalanalysistime(min)Total analysis time (min) |
|
|
1 |
82.0 |
0.7 |
9.027 |
11.944 |
1.445 |
1.567 |
1.384 |
6.975 |
|
2 |
74.9 |
0.7 |
10.326 |
17.989 |
1.385 |
1.404 |
1.230 |
10.077 |
|
3 |
82.0 |
0.5585 |
9.582 |
12.238 |
1.454 |
1.697 |
1.498 |
9.267 |
|
4 |
82.0 |
0.8414 |
8.590 |
11.104 |
1.470 |
1.660 |
1.474 |
5.686 |
|
5 |
77.0 |
0.8 |
9.568 |
16.037 |
1.378 |
1.465 |
1.324 |
7.858 |
|
6 |
77.0 |
0.6 |
10.273 |
16.647 |
1.383 |
1.562 |
1.414 |
10.439 |
|
7 |
87.0 |
0.6 |
7.305 |
8.402 |
1.493 |
1.563 |
1.457 |
6.158 |
|
8 |
87.0 |
0.8 |
7.162 |
7.917 |
1.549 |
1.506 |
1.309 |
4.761 |
|
9 |
89.1 |
0.7 |
6.340 |
6.937 |
1.634 |
1.560 |
1.224 |
4.957 |
Table 2 Experimental runs given by CCD for the two variables at triplet levels and their observed values.
Design Expert 9.0 software was employed for RSM computations to produce polynomial models. In the process of analysing the selected model, they were initially evaluated for the fit summary, which gives associability between variables and the responses. The statistical parameters from the analysis of variance (ANOVA) results for this method were listed in Table 3. The values for the model were taken with no transformation. The model terms with probability (Prob > F) ( value) < 0.05 were all highly significant. The high values of the adjusted R2 for the model reveals that there is a close relation between the experimental and the predicted values of the responses there by indicating the significance and the predictableness of the model. Linear factor equation The independent variable effects and their interaction influences were studied from the computer generated polynomial regression equations (Eq. (1–6)) and given as:
|
Variables |
Rs(HTZ−IRB) |
Rs(IRB−CST) |
THTZ |
TIRB |
TCST |
Totalanalysistime(min)Total analysis time (min) |
||||||
|
F |
P |
F |
P |
F |
P |
F |
P |
F |
P |
F |
P |
|
|
Model |
287.08 |
< 0.0001 |
1084.06 |
< 0.0001 |
31.61 |
< 0.0001 |
6.47 |
0.0148 |
17.85 |
0.0007 |
236.34 |
< 0.0001 |
|
A–% Organic phase |
1288.15 |
<0.0001 |
5349.91 |
<0.0001 |
62.38 |
<0.0001 |
5.20 |
0.0566 |
0.054 |
0.8223 |
834.19 |
< 0.0001 |
|
B–Flow rate |
53.82 |
0.0002 |
38.06 |
0.0005 |
0.84 |
0.3800 |
3.21 |
0.1164 |
10.55 |
0.0141 |
319.15 |
< 0.0001 |
|
AB |
6.70 |
0.0360 |
0.16 |
0.6982 |
– |
– |
0.24 |
0.6384 |
0.96 |
0.3598 |
10.94 |
0.0130 |
|
A2 |
85.45 |
<0.0001 |
27.13 |
0.0012 |
– |
– |
13.42 |
0.0080 |
43.05 |
0.0003 |
10.78 |
0.0134 |
|
B2 |
0.00785 |
0.9319 |
2.39 |
0.1664 |
– |
– |
7.29 |
0.0306 |
24.79 |
0.0016 |
8.91 |
0.0204 |
|
Adj R2* |
0.9917 |
0.9978 |
0.8361 |
0.6950 |
0.8753 |
0.9899 |
||||||
|
Adeq. Precision** |
55.926 |
107.664 |
16.441 |
10.099 |
15.443 |
48.656 |
||||||
Table 3 Analysis of variance for the screened chromatographic responses.
F – Fisher ratio; P – Probability.
Where, is % organic phase (%ACN) and is flow rate (ml/ min).
The positive value in the equation indicates the favourable response and negative value indicates the inverse effect among the variable and the response respectively. From the equations it is clear that the % organic phase (A) has positive effect on resolution of IRB, resolution of CST and total run time. Whereas, flow rate shows positive effect on resolution of HTZ. On the other hand, the other responses show the mixed type of response.
The perturbation plots (Figure 3a–3f) as well as the three–dimensional (3D) response surface plots (Figure 4a–4f) are very useful for studying the interactions effects of the variables on the responses. The relation between the response variables (i.e.,;; and ; and the independent variables is quadratic while in case of it is linear.
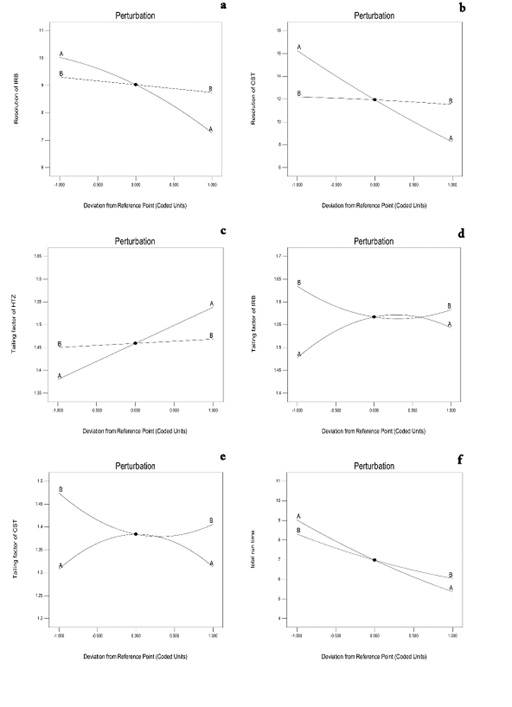
Figure 3 Perturbation plots representing the effect of % organic phase (A) and flow rate (B) on (a) Rs(HTZ-IRB) (b) Rs(IRB-CST) (c) THTZ (d) TIRB (e) TCST (f) Total analysis time.
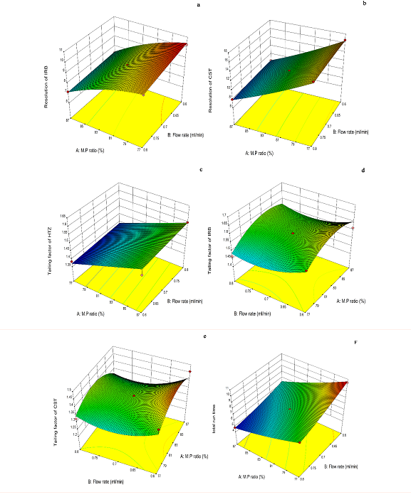
Figure 4 3D Response surface plots representing the effect of % organic phase (A) and flow rate (B) on (a) Rs (HTZ-IRB) (b) Rs (IRB-CST) (c) THTZ (d) TIRB (e) TCST (f) Total analysis time.
The final composition independent variables for the optimization of the HPLC method was achieved by conciliating the various responses (Table 4) to obtain better resolution among the peaks with good tailing factor and minimum analysis time. The bar graph for the desirability in the optimization was shown in Figure 5. Whereas, the desirability ramp for this method was shown in Figure 6 in which the limits for the variables and the desirability criteria were clearly shown. The graphical examination of the desirability was:
|
Name |
Goal |
Lower Limit |
Upper Limit |
|
Rs(HTZ−IRB) |
maximize |
6.34 |
10.326 |
|
Rs(IRB−CST) |
maximize |
6.937 |
17.989 |
|
THTZ |
minimize |
1.378 |
1.634 |
|
TIRB |
minimize |
1.404 |
1.697 |
|
TCST |
minimize |
1.224 |
1.498 |
|
Totalanalysistime(min)Total analysis time (min) |
minimize |
4.761 |
10.439 |
Table 4 Desirability criteria for optimized individual responses.
Total 10 runs are given for the design expert software having better desirability which were experimented and the percentage prediction error (P.E) were calculated as per Eq. 7 and shown in Table 5. The run for which the mean percentage prediction error is least compared to the other runs is concluded as the desired method.
|
Solution |
% Organic Phase |
Flow Rate |
Responses |
Predicted Values |
Observed Values |
PE |
Mean PE |
|
1 |
77.000 |
0.7599 |
Rs(HTZ−IRB) |
9.769 |
9.89 |
1.237 |
2.666 |
|
Rs(IRB−CST) |
15.995 |
15.916 |
–0.496 |
||||
|
THTZ |
1.386 |
1.369 |
–1.204 |
||||
|
TIRB |
1.471 |
1.516 |
3.050 |
||||
|
TCST |
1.316 |
1.459 |
10.839 |
||||
|
Totalanalysistime(min)Total analysis time (min) |
8.243 |
8.455 |
2.570 |
||||
|
2 |
77.000 |
0.7607 |
Rs(HTZ−IRB) |
9.766 |
9.829 |
0.645 |
2.162 |
|
Rs(IRB−CST) |
15.992 |
15.896 |
–0.600 |
||||
|
THTZ |
1.386 |
1.370 |
–1.136 |
||||
|
TIRB |
1.471 |
1.492 |
1.411 |
||||
|
TCST |
1.317 |
1.455 |
10.506 |
||||
|
Totalanalysistime(min)Total analysis time (min) |
8.234 |
8.411 |
2.144 |
||||
|
3 |
77.000 |
0.7587 |
Rs(HTZ−IRB) |
9.775 |
9.926 |
1.549 |
2.673 |
|
Rs(IRB−CST) |
16.001 |
16.040 |
0.241 |
||||
|
THTZ |
1.386 |
1.376 |
–0.690 |
||||
|
TIRB |
1.471 |
1.508 |
2.518 |
||||
|
TCST |
1.316 |
1.440 |
9.445 |
||||
|
Totalanalysistime(min)Total analysis time (min) |
8.258 |
8.504 |
2.976 |
||||
|
4 |
77.000 |
0.7571 |
Rs(HTZ−IRB) |
9.781 |
10.018 |
2.422 |
2.171 |
|
Rs(IRB−CST) |
16.009 |
16.128 |
0.745 |
||||
|
THTZ |
1.385 |
1.382 |
–0.247 |
||||
|
TIRB |
1.471 |
1.467 |
–0.256 |
||||
|
TCST |
1.315 |
1.408 |
7.070 |
||||
|
Totalanalysistime(min)Total analysis time (min) |
8.277 |
8.549 |
3.292 |
||||
|
5 |
77.000 |
0.7564 |
Rs(HTZ−IRB) |
9.784 |
10.076 |
2.982 |
1.813 |
|
Rs(IRB−CST) |
16.012 |
16.009 |
–0.020 |
||||
|
THTZ |
1.385 |
1.376 |
–0.675 |
||||
|
TIRB |
1.471 |
1.446 |
–1.678 |
||||
|
TCST |
1.315 |
1.405 |
6.868 |
||||
|
Totalanalysistime(min)Total analysis time (min) |
8.285 |
8.567 |
3.399 |
||||
|
6 |
77.000 |
0.7642 |
Rs(HTZ−IRB) |
9.751 |
9.75 |
–0.010 |
1.402 |
|
Rs(IRB−CST) |
15.975 |
15.962 |
–0.081 |
||||
|
THTZ |
1.386 |
1.366 |
–1.448 |
||||
|
TIRB |
1.472 |
1.457 |
–1.006 |
||||
|
TCST |
1.318 |
1.437 |
8.992 |
||||
|
Totalanalysistime(min)Total analysis time (min) |
8.193 |
8.354 |
1.964 |
||||
|
7 |
77.000 |
0.7548 |
Rs(HTZ−IRB) |
9.791 |
10.216 |
4.341 |
1.123 |
|
Rs(IRB−CST) |
16.020 |
16.060 |
0.252 |
||||
|
THTZ |
1.385 |
1.368 |
–1.242 |
||||
|
TIRB |
1.471 |
1.391 |
–5.407 |
||||
|
TCST |
1.314 |
1.379 |
4.944 |
||||
|
Totalanalysistime(min)Total analysis time (min) |
8.304 |
8.624 |
3.848 |
||||
|
8* |
77.000 |
0.7531 |
Rs(HTZ−IRB) |
9.798 |
10.332 |
5.447 |
–0.074 |
|
Rs(IRB−CST) |
16.028 |
16.065 |
0.233 |
||||
|
THTZ |
1.385 |
1.365 |
–1.448 |
||||
|
TIRB |
1.470 |
1.326 |
–9.818 |
||||
|
TCST |
1.313 |
1.329 |
1.193 |
||||
|
Totalanalysistime(min)Total analysis time (min) |
8.325 |
8.654 |
3.950 |
||||
|
9 |
77.000 |
0.7735 |
Rs(HTZ−IRB) |
9.711 |
9.45 |
–2.688 |
0.751 |
|
Rs(IRB−CST) |
15.929 |
15.844 |
–0.532 |
||||
|
THTZ |
1.387 |
1.366 |
–1.509 |
||||
|
TIRB |
1.474 |
1.466 |
–0.532 |
||||
|
TCST |
1.324 |
1.429 |
7.945 |
||||
|
Totalanalysistime(min)Total analysis time (min) |
8.086 |
8.233 |
1.821 |
||||
|
10 |
77.000 |
0.7149 |
Rs(HTZ−IRB) |
9.960 |
10.839 |
8.822 |
0.569 |
|
Rs(IRB−CST) |
16.192 |
16.149 |
–0.265 |
||||
|
THTZ |
1.382 |
1.369 |
–0.907 |
||||
|
TIRB |
1.473 |
1.405 |
–4.630 |
||||
|
TCST |
1.306 |
1.275 |
–2.394 |
||||
|
Totalanalysistime(min)Total analysis time (min) |
8.817 |
9.063 |
2.786 |
Table 5 Table for optimization.
From this a combination of 0.1% acetic acid and acetonitrile (33:77, v/v) as mobile phase (Isocratic mode) with a flow of 0.7531 ml/min achieves the desirable responses. At this condition, of 10.332, of 16.065, of 1.365, of 1.326, of 1.329, and total analysis time of 8.654 were observed and the chromatograph was shown in Figure 7.
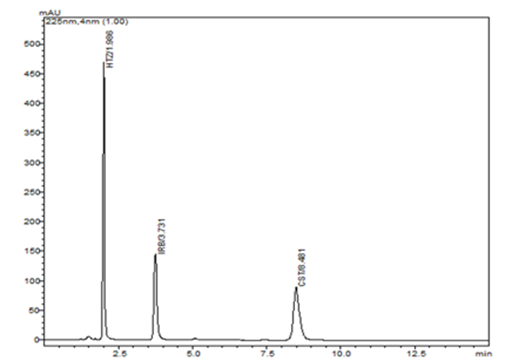
Figure 7 The desirability ramp representing the optimization of the independent variables for the better responses.
Method validation
The method was validated for system suitability, linearity, accuracy, precision, limit of detection (LOD), limit of quantitation (LOQ), selectivity and robustness (ICH guidelines, 2005).
Linearity
HTZ, IRB and CST follows linearity over a concentration range of 0.01–60µg/ml, 0.05 –120µg/ml and 0.05–100µg/ml respectively (Table 6) with % RSD 0.11–0.45, 0.08–0.42 and 0.10–0.65 for HTZ, IRB and CST respectively. The chromatographic responses of individual samples were shown in Figure 8C–8E respectively for HTZ, IRB and CST respectively. The linear regression equations were found to be y = 198986x –11128 (r2 = 0.9999), y= 100726x–5887.5 (r2 = 0.9997) y=117644x+27207 (r2 = 0.9999) for HTZ, IRB and CST respectively. The limit of detection (LOD) and limit of quantitation (LOQ) of each drugs were shown in Table 6.
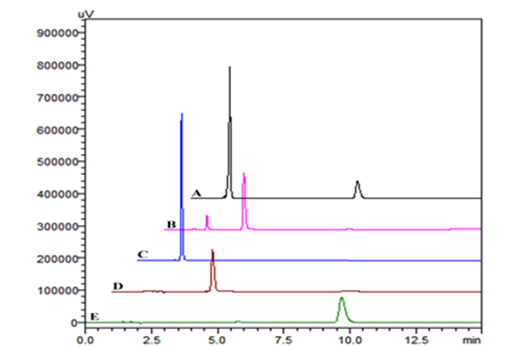
Figure 8 Typical chromatograms [A] CANDESAR-H®, [B] IROVEL–H®, [C]HTZ (10μg/ml), [D] IRB (10μg/ml) and[E] CST (10μg/ml) .
|
Statistical Parameters |
Data* |
||
|
HTZ |
IRB |
CST |
|
|
Linearity range (µg/ml) |
0.01–60 |
0.05 –120 |
0.05 – 100 |
|
Correlation coefficient (R2) |
0.9999 |
0.9997 |
0.9999 |
|
Slope of curve |
198986 |
100726 |
117644 |
|
Intercept of curve |
11128 |
5887.5 |
27207 |
Table 6 Linear regression data for the calibration curve.
*Mean of three replicates.
Precision
The intra–day precision of the method was established by assaying the samples of HTZ, IRB and CST at three different concentration levels i.e., 5,10 and 20μg/ml for HTZ and 10, 20 and 50μg/ml for IRB and CST on the same day. The inter–day precision was calculated by assaying the samples of HTZ, IRB and CST at three different concentration levels i.e., 5,10 and 20μg/ml for HTZ and 10,20 and 50μg/ml for IRB and CST on three different days. The % RSD for intra–day precision was found to be 0.06–0.61, 0.34–0.54 and 0.02–0.08 respectively for HTZ, IRB and CST. Whereas the% RSD for inter–day precision was found to be 0.65–1.32, 0.61–1.25 and 0.24–1.19 for HTZ, IRB and CST respectively (Table 7).
|
Analyte |
Conc. (µg mL–1) |
Intra–Day Precision |
Inter–Day Precision |
|||||||
|
*Measured conc. (µg mL–1) |
%RSD |
SEM |
*Measured conc. (µg mL–1) ± SD |
%RSD |
SEM |
|||||
|
HTZ |
5 |
5.02±0.03 |
0.61 |
0.0177 |
4.93±0.06 |
1.32 |
0.0375 |
|||
|
10 |
10.00±0.01 |
0.08 |
0.0046 |
9.89±0.11 |
1.10 |
0.0629 |
||||
|
20 |
20.00±0.01 |
0.06 |
0.0065 |
19.90±0.13 |
0.65 |
0.0751 |
||||
|
IRB |
10 |
9.82±0.04 |
0.45 |
0.0257 |
9.87±0.12 |
1.25 |
0.0715 |
|||
|
20 |
19.54±0.10 |
0.54 |
0.0606 |
19.86±0.13 |
0.66 |
0.0752 |
||||
|
50 |
49.73±0.17 |
0.34 |
0.0981 |
49.67±0.30 |
0.61 |
0.1760 |
||||
|
CST |
10 |
10.03±0.01 |
0.08 |
0.0046 |
9.88±0.12 |
1.19 |
0.0678 |
|||
|
20 |
20.02±0.01 |
0.02 |
0.0025 |
19.83±0.15 |
0.77 |
0.0879 |
||||
|
50 |
50.01±0.02 |
0.05 |
0.0138 |
49.86±0.12 |
0.24 |
0.0688 |
||||
|
Accuracy Studies |
||||||||||
|
Analyte |
Spiked Conc. |
Total Theoretical conc. |
*Conc. Found (µg mL–1) |
%RSD |
SEM |
%Recovery |
||||
|
HTZ |
4 (80 %) |
9 |
8.85±0.0318 |
0.36 |
0.2042 |
98.31 |
||||
|
5 (100 %) |
10 |
9.91±0.0810 |
0.82 |
0.4677 |
99.12 |
|||||
|
6 (120 %) |
11 |
10.81±0.0889 |
0.82 |
0.4668 |
98.23 |
|||||
|
IRB |
8 (80 %) |
18 |
17.80±0.0602 |
0.34 |
0.1929 |
98.88 |
||||
|
10 (100 %) |
20 |
19.96±0.0496 |
0.25 |
0.1433 |
99.79 |
|||||
|
12 (120 %) |
22 |
21.85±0.0305 |
0.14 |
0.0802 |
99.34 |
|||||
|
CST |
8 (80 %) |
18 |
17.78±0.0422 |
0.24 |
0.1353 |
98.78 |
||||
|
10 (100 %) |
20 |
19.80±0.0644 |
0.32 |
0.1858 |
99.02 |
|||||
|
12 (120 %) |
22 |
21.74±0.0193 |
0.09 |
0.0507 |
98.83 |
|||||
Table 7 Precision and accuracy studies of HTZ, IRB and CST.
*Mean of triplet replicates.
Accuracy
The method accuracy was proved by the recovery test at three different concentrations (80, 100 and 120 %) for all the three drugs. A known amount of standards (10μg/ml) were added to sample solutions and then further diluted to achieve the total concentrations of 18, 20 and 22μg/ml for all the three drugs as described in Table 7. The % recovery for HTZ, IRB and CST was found to be 98.23–99.12, 98.88–99.79 and 98.78–99.02 respectively with %RSD with in acceptance criteria (<2.0 %).
Robustness
The robustness of an analytical procedure is referred as the method’s ability to retain unaffected even by small variations in parameters from the original conditions and there by proves the ability of its reliability for routine analysis. The detection wavelength was set at 223 and 227nm (±2nm), the ratio of percentage of 0.1% acetic acid: acetonitrile in the mobile phase was applied as 25:75 and 21:79 (±2, v/v), the flow rate was set at 0.6531 and 0.8531 ml/min (±0.1ml/min). The results obtained for the robustness study were shown in Table 8. From the results it was shown that the retention times and the assays for the test solutions were not much affected by varying the conditions and were in consonance with the results for original conditions. The % RSD value of assay of the sample under original conditions and robustness conditions was less than 2.0% (i.e., 0.018–0.322 for Rt and for assay it is 0.0292–0.2152) indicating that the method is robust.
|
Parameter (Condition) |
Analyte |
*%Assay ± SD |
%RSD |
SEM |
*Retention Time ± SD |
%RSD |
SEM |
|
Mobile Phase Flow Rate (± 0.1 mL.min–1) |
|||||||
|
(0.6531 mL.min–1) |
HTZ |
98.96±0.0557 |
0.0563 |
0.0322 |
2.019±0.007 |
0.322 |
0.0038 |
|
IRB |
98.36±0.1810 |
0.1840 |
0.1045 |
3.844±0.003 |
0.084 |
0.0019 |
|
|
CST |
98.48±0.0351 |
0.0356 |
0.0202 |
8.594±0.003 |
0.029 |
0.0015 |
|
|
(0.8531 mL.min–1) |
HTZ |
99.34±0.111 |
0.1120 |
0.0642 |
1.922±0.005 |
0.235 |
0.0026 |
|
IRB |
101.19±0.1006 |
0.0994 |
0.0581 |
3.672±0.002 |
0.042 |
0.0009 |
|
|
CST |
100.20±0.063 |
0.0627 |
0.0363 |
8.354±0.004 |
0.042 |
0.0020 |
|
|
Detection Wavelength (±2nm) |
|||||||
|
(223 nm) |
HTZ |
100.34±0.029 |
0.0292 |
0.0169 |
1.985±0.003 |
0.154 |
0.0018 |
|
IRB |
101.94±0.0436 |
0.0427 |
0.0251 |
3.733±0.002 |
0.056 |
0.0012 |
|
|
CST |
100.80±0.215 |
0.2128 |
0.1239 |
8.483±0.002 |
0.025 |
0.0012 |
|
|
(227 nm) |
HTZ |
98.53±0.076 |
0.0774 |
0.0440 |
1.985±0.003 |
0.127 |
0.0015 |
|
IRB |
98.48±0.0546 |
0.0555 |
0.0315 |
3.731±0.003 |
0.086 |
0.0019 |
|
|
CST |
99.32±0.068 |
0.0686 |
0.0393 |
8.483±0.002 |
0.025 |
0.0012 |
|
|
Mobile Phase Composition (±2 % Acetonitrile, v/v) |
|||||||
|
(25:75, v/v) |
HTZ |
99.98±0.140 |
0.1397 |
0.0807 |
1.995±0.002 |
0.077 |
0.0009 |
|
IRB |
100.15±0.1444 |
0.1441 |
0.0833 |
3.823±0.002 |
0.040 |
0.0009 |
|
|
CST |
9953±0.120 |
0.1210 |
0.0695 |
8.484±0.002 |
0.018 |
0.0009 |
|
|
(21:79, v/v) |
HTZ |
99.90±0.191 |
0.1909 |
0.1101 |
1.973±0.004 |
0.183 |
0.0021 |
|
IRB |
99.45±0.2140 |
0.2152 |
0.1236 |
3.682±0.002 |
0.041 |
0.0009 |
|
|
CST |
101.06±0.103 |
0.1022 |
0.0596 |
8.472±0.002 |
0.018 |
0.0009 |
|
Table 8 Robustness Studies of HTZ, IRB and CST.
*Mean of triplet replicates.
Analysis of commercial formulations
The proposed method was applied to the available marketed formulations i.e., IROVEL–H® (label claim: 150mg/12.5mg of IRB/HTZ), and CANDESAR–H® (label claim: 16mg/12.5mg of CST/HTZ). The % recovery was found to be 97.69–98.37% for HTZ, 98.47% for IRB and 98.51% for CST (Table 9). The resultant chromatograms obtained for marketed formulations were shown in Figure 8.
A simple isocratic RP–HPLC method for simultaneous determination of HTZ, IRB and CST in bulk and markedly available pharmaceutical dosage form was developed. For this central composite design which is a response surface methodology was adapted to spot out the significant impact of the independent variables such as % organic phase and the flow rate each at triplet levels on the chromatographic responses. The chromatographic responses such at the resolution, theoretical plates, tailing factor and total analysis time were simultaneously optimized with the backing of design of experiments methodology. This multivariate chemometric assisted experimental method development and its validation emphasizes that systematic approach for quality leads to creation of highly budgetary and conscientious chromatographic methods.
The authors are grateful to University Grants Commission, New Delhi for their financial support, Sun Pharmaceutical Industries Ltd., (India) for providing the gift samples and M/s GITAM University, Visakhapatnam for providing the research facilities.
None.

©2017 nnapurna, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.