
Research Article Volume 4 Issue 1
The Inhibitive Action of Aniline on the Autoxidation of Sodium Sulfite in Acidic Medium
Arun Kumar Sharma,
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Parashar P, Prasad DSN, Rashmi Sharma
Correspondence: Arun Kumar Sharma, Department of Chemistry, Govt. P.G. College , Jhalawar, 326001, Rajasthan, India
Received: December 05, 2016 | Published: January 19, 2017
Citation: Sharma AK, Parashar P, Prasad DSN, Sharma R (2017) The Inhibitive Action of Aniline on the Autoxidation of Sodium Sulfite in Acidic Medium. J Anal Pharm Res 4(1): 00091. DOI: 10.15406/japlr.2017.04.00091
Download PDF
Abstract
The kinetics of atmospheric autoxidation of S (IV) by Ag(I) at pH 4.02-5.25 has been studied. The aqueous phase autoxidation of S (IV) is the major factor responsible for acidification of atmospheric aqueous system. The role of Aniline act as an inhibitor of Ag(I) catalysed autoxidation of S(IV) in acidic medium has been identified, and based on the observed results following rate law given and a free radical mechanism has been proposed.
Experiments were carried out at 30≤T°C≤40, 4.02≤pH≤5.25, 1×10−3mol/cm3≤ [S(IV)≤10×10−3 mol/cm3, 5×10−6mol/cm3≤[Ag(I)]≤2.5×10−5mol/cm3, 5×10−7mol/cm3≤[Aniline]≤8×10-3mol/cm3. Based on the experimental results, rate constants and orders of the reactions were determined. The reaction order in S(IV) were first order for both reactions in the presence and absence of Aniline. The effect of Ag(I) ion and Aniline concentrations as well as an initial pH of the solution on the S(IV) oxidation rate were discussed. It was found that the rate of the S(IV) oxidation depends on the initial pH of the solution but it is independent of the pH change during the reaction. Addition of Aniline leads to the introduction of an induction period and decrease in reaction rate, most likely due to SO4-1 radicals. The value of apparent energy and inhibition constant B were calculated in the presence of Aniline found as 26.43 KJ mol-1and 0.26x103mol dm-3 respectively.
Keywords: kinetics, autoxidation, s(iv), ag(i), catalysis, inhibition, aniline, oxidation, concentrations
Introduction
Although the SO2 oxidation by O2 has been the subject of many studies, the mechanism of the reaction is far for settled. Both non radical and radical mechanism have been proposed. The atmospheric oxidation of S02 by 02 in aqueous media has been the subject of numerous studies, and the subject matter of several reviews, monographs and papers, notably by Kuo et al.,1‒11 It is interesting to point out that reaction is strongly inhibited by alcohol, benzene, and other compounds. Pointing to the participation of radical intermediates.12‒17 Bigelow18 was the first to observe the inhibiting effect of alcohols such as methanol, ethanol, propanol, butanol on the rate of the reaction between sodium sulfite and oxygen. The inhibiting effect of alcohols was investigated subsequently by Alyea et al.,19 The other Organics studied are phenols20 organic acids,21‒23 benzene,24 toluene, naphthalene, paraffin oil,25 alpha-pinene, cis – verbenol,26 sobrerol,27 myrtenol.28 The effect of aromatic amines i.e. aniline in atmospheric water on the transition metal-catalysed oxidation of S(IV) is not fully known yet and more work in this area is needed to understand these processes better. The purpose of the present study was to study the kinetics of the Ag(I)catalysed S(IV) oxidation and to determine the inhibiting effect of aniline on this process under different experimental conditions.
Experimental
The experimental procedure was exactly the same as described earlier.29 All the chemicals used were AR grade and their solutions were prepared in doubly distilled water. The reaction were conducted in 0.15L Erlenmeyer flask, open to air and follow to passage of atmosphere oxygen. The flask was placed in a beaker which had an inlet at a lower part and an outlet at a outer part for circulating thermostatic water for maintaining the desired temperature 30+10C. The reaction was initiated by adding the desired volume of Na2S03 solution to the reaction mixture containing other additive such as buffer and catalyst. The reaction mixture was stirred continuously and magnetically at 1600+10rpm to allow the passage of atmospheric oxygen and to save the reaction from becoming oxygen mass transfer controlled. The kinetics was studied in acetate buffered medium in which the pH remained fixed throughout the entire course of reaction. For this purpose 10cm3 buffer made from sodium acetate (0.07mol L-1) and acetic acid (0.03mol L-1) for acidic medium were used (total volume 100cm3) for obtaining the desired pH. The kinetics were followed by withdrawing the aliquot samples periodically and titrating the unreacted S(IV) iodometrically. The reproducibility of replicate measurements was generally better than 10+1 %. All calculations were performed in MS Excel.
Product analysis
The qualitative test shows sulphate to be only oxidation product. For quantitative analysis, the reaction mixture containing catalyst and S(IV) in appropriate buffered solutions were constantly stirred for a sufficiently long time so as to ensure complete oxidation of S(IV). When the reaction was complete then S(VI) estimated gravimetrically by precipitating sulphate ions as BaSO4 using standard procedure.
The product analysis showed the recovery of sulphate to be 98+1%, in all cases in agreement with eq. (1)
(1)
Result
Preliminary investigation
The kinetics of both uncatalysed and Ag(I) catalysed and aniline inhibited reaction were studied in acidic medium in pH 4.95 and temperature 30°C. In both the cases the first order dependence of S(IV) was observed in the kinetics data treatment for the determination of first order rate constant k1 was carried out from log [S(IV)] versus time, t. The plots were shown in Figure 1 It is obs. from Figure 1 that both the uncatalysed and Ag(I) catalysed autoxidation of S(IV) reaction are inhibited by aniline.
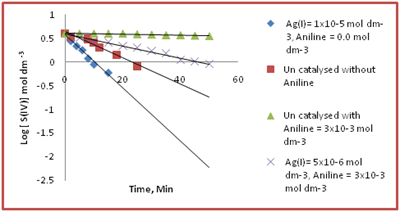
Figure 1 The disappearance of [S(IV)] with time in air saturated suspensions at [S(IV)] =2x 10-3 mol dm-3 at pH = 4.95, t = 30°C.
◊ Ag (I) = 1x 10-5mol dm-3, [Aniline] = 0.0 mol dm-3.
□ Uncatalysed without aniline.
△ Uncatalysed with [Aniline] = 3x10-3 mol dm-3.
x Ag (I) = 5x 10-6mol dm-3, [Aniline] = 3x 10-3mol dm-3.
Uncatalysed reaction
Uncatalysed reaction was studied in the absence of Ag(I) and all the solutions were prepared in doubly distilled water.
Dependence of S(IV)
The detail dependence of the reaction rate on [S(IV)] was studied by varying it is in the range 1x10-3mol dm-3 to 4x10-3mol dm-3 at pH = 4.95, t = 30°C in acetate buffered medium. The kinetics was found to be first order in [S(IV)] and values of K1 was calculated from log [S(IV)] v/s time plots which was linear. The values of first order rate constant K1 are given in Table 1. The dependence of reaction rate on [S(IV)] follows the rate law (2).
|
[S(IV)] mol dm-3
|
(103) K1 s-1
|
|
0.001
|
1.04
|
|
0.002
|
1.06
|
|
0.003
|
1.09
|
|
0.004
|
1.09
|
Table 1 The values of K1 for uncatalysed reaction at different [S(IV)], pH = 4.95, t = 30°C, CH3COONa=7x10-2mol L-1, CH3COOH= 3x10-2mol L-1
(2)
[Aniline] dependence
The major aim of this study was to examine the effect of aniline on the autoxidation of S(IV) in acetate buffer medium and varying the [aniline] from 5x10-7mol dm-3 to 8x10-3mol dm-3, we observed the rate of thereaction decreased by increasing [aniline] The results are given in Table 2 However the nature of the [S(IV)] dependence in presence of aniline did not change and remains first order. The first order rate constant Kinh in the presence of anilinewas defined by rate law (3).
|
[Aniline] mol dm-3
|
103 Kinh s-1
|
1/Kinh s
|
|
5.0x10-7
|
0.94
|
1064
|
|
5.0x10-6
|
0.827
|
1209
|
|
8.0x10-6
|
0.707
|
1414
|
|
1.0x10-5
|
0.607
|
1647
|
|
5.0x10-5
|
0.513
|
1949
|
|
8.0x10-5
|
0.418
|
2392
|
|
1.0x10-4
|
0.36
|
2778
|
|
5.0x10-4
|
0.284
|
3521
|
|
8.0x10-4
|
0.189
|
5291
|
|
3.0x10-3
|
0.138
|
7246
|
|
5.0x10-3
|
0.075
|
13333
|
|
8.0x10-3
|
0.037
|
27027
|
Table 2 The values of Kinh at different [Aniline], pH = 4.95, t = 30°C CH3COONa =7x10-2mol L-1 CH3COOH= 3x10-2mol L-1
(3)
The values of Kinh in the presence of aniline decreased with increasing [Aniline] are given in Table 2 which are in agreement with the rate law (4).
(4)
Where B is inhibition parameter for rate inhibition by aniline. The equation (4) on rearrangement becomes
(5)
In accordance with the equation (5) the plot of 1/ Kinh v/s [aniline] was found to be linear with non- zero intercept. The values of intercept (1/ K1) and slope (B/ K1) were found to be 1.53 x 103s and 2.86 x 106 mol dm-3s at pH = 4.95, t = 30°C From these values the value of inhibition parameter B was found to be 1.86x 103mol dm-3.
Ag(I) catalysed reaction
At first the kinetics of Ag(I) Catalysed reaction in the absence of inhibitor was studied.
[S(IV)] variation
The dependence of S (IV) on reaction rate was studied by varying [S(IV)] from 1x10-3mol dm-3 to 10x10-3mol dm-3 at two different but fixed Ag(I) of 5x10-6mol dm-3and 1x10-5mol dm-3 at pH = 4.95, t = 30°C The kinetics was found to be first order in [S(IV)] v/s time were linear as shown in Figure 1.
Ag(I) variations
The dependence of Ag(I) on the reaction rate was studied by varying Ag (I) from 5x10-6mol dm-3 to 2.5x10-5moldm-3at S(IV) = 2x10-3mol dm-3 pH= 4.95, t= 30°C in acetate buffer medium. The values of First order rate constant kcat for S(IV) oxidation was determine are shown in Figure 2. The nature of dependence of kcat on Ag (I) was indicated as two term rate law (6).
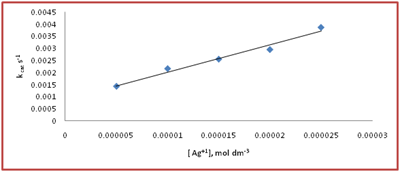
Figure 2 The dependence of catalyst concentration at [S(IV)] = 2x10-3 mol dm-3, pH = 4.95, t = 30°C in acetate buffered medium.
(6)
Or
(7)
From the plot in Figure 2 the values of intercept is equal to K1 and slope is equal to k2 were found to be 0.72x 101 s and 8.6 x 10-3 mol dm-3 s respectively at pH = 4.95, t = 30°C in acetate buffered medium.
Variation of pH
Variation of pH was carried out from 4.02- 5.25 at different [S (IV), Ag (I), [Aniline] and temperatures. The rate decreases slightly by varying pH is inverse H+ ion dependence was observed. From the plot of log K1 v/s log (H+) the order with respect to H+ is 0.16 which is a fractional order and can be neglected as shown in Figure 3 (Table 3 & 4).
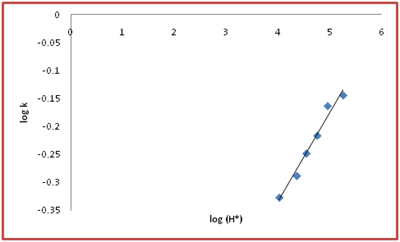
Figure 3 Effect of pH at [S(IV)] = 2x10-3 mol dm-3 ,Ag (I) = 5x10-6 mol dm-3, [Aniline]= 5.0x10-4 mol dm-3, t= 30°C in acetate buffered medium.
|
[Aniline], mol dm-3
|
Ag (I) =5x10-6 mol dm-3
|
Ag (I) =1 x 10-5mol dm-3
|
Ag (I) =1.5 x 10-5
|
|
pH = 4.02
|
|
|
|
|
5.0 x 10-4
|
0.470 x 10-3
|
0.564 x 10-3
|
0.717 x 10-3
|
|
8.0x 10-4
|
0.400 x 10-3
|
_
|
_
|
|
3.0 x 10-3
|
0.319 x 10-3
|
_
|
_
|
|
pH = 4.50
|
|
|
|
|
5.0 x 10-4
|
0.499 x 10-3
|
0.590 x 10-3
|
0.788 x 10-3
|
|
8.0 x 10-4
|
0.471 x 10-3
|
_
|
_
|
|
3.0 x 10-3
|
0.402 x 10-3
|
_
|
_
|
|
pH = 5.25
|
|
|
|
|
5.0 x 10-4
|
0.586 x 10-3
|
0.718 x 10-3
|
0.942 x 10-3
|
|
8.0 x 10-4
|
0.577 x 10-3
|
_
|
_
|
|
3.0 x 10-3
|
0.516 x 10-3
|
_
|
_
|
Table 3 Rate of Ag (I) catalysed autoxidation in the presence of Aniline
|
[Aniline] 5.0 x 10-4 mol dm-3
|
Ag (I) =5x10-6 mol dm-3
|
Ag (I) =1 x 10-5 mol dm-3
|
Ag (I) =1.5 x 10-5 mol dm-3
|
|
pH = 4.50
|
|
|
|
|
|
3.02
|
3.82
|
3.54
|
Table 4 Ratio of rates for Ag (I) catalysed oxidation in the absence and in the presence of Aniline
[Aniline] dependence
To know the effect of aniline on Ag(I) catalysed autoxidation of S(IV) aniline variation was carried out from 5x10-7mol dm-3 to 3 x 10-3 mol dm-3 at two different Ag (I) that is 5x10-6mol dm-3 to 1 x 10-5mol dm-3 but fixed S(IV) = 2x10-3mol dm-3, pH= 4.95, t= 30°C The results indicated that by increasing aniline the rate becomes decelerates (Figure 4 & 5).
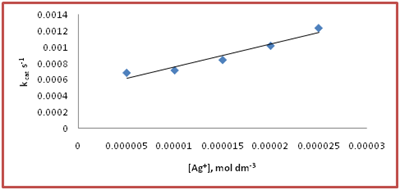
Figure 4 Effect of catalyst at [S(IV)] = 2x10-3mol dm-3, [Aniline]= 5.0x10-4mol dm-3, t= 30°C in acetate buffered medium. The value of intercept and slope are 4.8 x 10-4 s and 2.7x101 mol dm-3 s respectively. Depending on the observed results the reaction follows the following rate law (8).
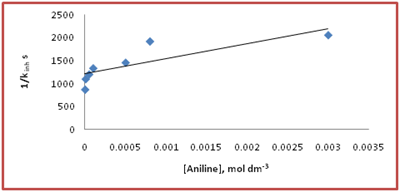
Figure 5 Effect of aniline at [S(IV)] = 2x10-3 mol dm-3, Ag (I) = 5x10-6 mol dm-3, pH = 4.95, t = 30°C in acetate buffered medium. The plot of 1/ Kinh v/s [Aniline] is linear with intercept 1.21 x 103s and slope 3.25x105 mol dm-3 s from which the value of B = 0.26 x103 mol dm-3.
(8)
Where
(9)
(10)
(11)
Effect of temperature
The values of kobs were determined at three different temperatures in the range of 30°C to 40°C. The results are given in Table 5. By plotting a graph between log k v/s 1/T yield us an apparent empirical energy of activation 26.43 KJ moL-1.
|
toC
|
103 kobs S-1
|
|
30
|
0.686
|
|
35
|
0.781
|
|
40
|
0.915
|
Table 5 Effect of temperature kobs air saturated suspensions at [S(IV)] = 2x10-3mol dm-3, Ag (I) = 5 x10-6mol dm-3, [Aniline] = 5.0 x 10-4mol dm-3, pH = 4.95
Discussion
In aqueous solutions S02 is present in four forms S02.H2O, HS03-1, S03-2, S203-2 In the experimental range of pH the following equilibrium operates
(12)
The equilibrium constant is 5.07x 10-7In the experimental range of pH both species HS03-1, S03-2 are present but former one present predominantly. During the course of reaction fraction order obtain is 0.18 indicates that it is almost independent of pH which is co-relate with the work of Irena.30 Prasad et al.,31 studied the inhibiting effect of formic acid,31 isopropyl alcohol,32 isoamyl alcohol33 in the presence of Ag(I) catalysed autoxidation of S(IV) and reported the they all are influence the S(IV) oxidation in atmosphere with moderate rate. Gupta et al.,34 studied the role of some organic organics on the oxidation of dissolved sulfur dioxide by oxygen in rain water medium and found it follows radical mechanism so free radical scavengers such as VOCs decelerate the S(IV) oxidation and control the rain water acidity.35 Bigelow et al.,18 studied the effect of alcohols on the reactions between sodium sulphite and 02 and found that the alcohols inhibited the reaction rate. Alyea et al.,19 studied the inhibiting effect of aliphatic alcohols on sodium sulphite in alkaline medium. Gupta et al.,34 studied the kinetics of environmentally important oxidation of S(IV) in acetate buffered medium in presence of Iron (III) in pH range 5.27- 5.70 and reported that addition of ethanol leads to decrease in reaction rate. Grgic et al.,36 studied about scavenging of SO4- radical anions by mono and dicarboxylic acids in the Mn(II) catalysed oxidation in aqueous solutions and reported that low molecular weight carboxylic acids have low reactivity towards sulphate radical anion. Backstrom19 proposed a radical chain mechanism for alcohol inhibited oxidation reaction between sodium sulphite and 02. Gupta et al studied the influence of hydroxyl VOCs on the oxidation of S(IV) by oxygen and found VOCs inhibited the S(IV) oxidation. Grgic et al.,36 studied the inhibition effect of acetate, oxlate, format on Fe- catalysed autoxidation of S(IV) at pH 2.8, 3.7,4.5 and found that oxlate has strong inhibiting effect on reaction rate due to the decrease amount of catalytic activity of Fe+3 due to formation of complexion with oxlate. Bostjan et al.,32 studied the effect of carboxylic acid on Mn(II) catalysed oxidation of S(IV) and found that monocarboxylic acid exhibit strong inhibition and out of which acetic acid shows strong inhibition. The rate of uncatalysed and Ag(I) catalysed reaction is decelerated by the addition of aniline in the present study. Gupta et al.,37 reported that radical mechanism operate in those reaction in which the inhibition parameters lies 103-104 In the present study the value of inhibition parameter for uncatalysed and Ag(I) catalysed autoxidation of S(IV) by anilineare found to be in the range 103-104 These values are in the same range as reported by Gupta et al.,37 This is strongly support the radical mechanism in the present case too based on the observed results. The free radical mechanism is very complex, the present work close to the experimental condition of Connick et al.,38
(13)
(14)
(15)
(16)
(17)
(18)
(19)
(20)
(21)
(22)
(23)
(24)
(25)
(26)
(27)
(28)
By assuming long chain hypothesis and steady state approximation d[S03]/dt, d[SO4]/dt, d[SO5]/dt, to zero. It can be shown that rate of initiation is equal to rate of termination (eq. 29).
(29)
Since the reaction is completely stopped in the presence of [Aniline] = 2x10-4mol dm-3, so the step (22) and (25) appear to be unimportant. The step (24) is ignored because the reaction is completely seized in the presence of higher concentration of Aniline by omission and substitution from the above mechanism the following rate law can be obtain (30).
(30)
Prasad & Gupta et al.,11,37 proposed a similar mechanism for the C0203 and CoO catalysed autoxidation of S02 inhibited by formic acid and ethanol respectively, which lead to the same rate law. By comparing derived rate law with the experimental rate law we observe the similarity in these two. The calculated value of inhibition constant B is 1.86x103mol dm-3 which is in the range of 103-104 and also coincide with the reported value of B of C0203 catalysed autoxidation of S(IV) by formic acid is 3.58 x 103 mol dm-3. So on the basis of calculated value of B we concluded that Aniline act as an free radical scavenger in Ag(I) catalysed autoxidation of aqueous S02 in acidic medium and a free radical mechanism can operate in this system.
Conclusion
The following conclusions are deduced from the results of the Aniline inhibited Ag(I) catalysed autoxidation of S(IV) was that inhibit the oxidation with the fast influence. The value of inhibition factor of both uncatalysed and Ag(I) catalysed autoxidation of S(IV) in the present study are in the range of 103-104 which shows that free radical mechanism is operative.
Acknowledgments
Conflicts of interest
Author declares there are no conflicts of interest.
Funding
References
- Kuo DTF, Krik DW, Jia CQ. The chemistry of aqueous S(IV)-Fe-O2 system: state of the art. Journal of Sulfur Chemistry. 2006;27(5):461‒530.
- Clemitshaw KC. Coupling between the trophospheric photochemistry of nitrous acid (HONO) and nitric acid (HNO3). Environmental Chemistry. 2006;3(1):31‒34.
- Brandt C, Van Eldik R. Transition metal-catalyzed oxidation of S(IV) oxides, Atmospheric relevant processes and mechanisms. Chemical Reviews. 1995;95(1):119‒180.
- Martin LR. Kinetic studies of sulphite oxidation in aqueous solution. In: Calvert JG (Eds.), Butterworth, Boston, USA. 1984.
- Manoj SV, Mudgak PK, Gupta SK. Kinetic of iron(III) catalysed autoxidation of S(IV) in acetate buffered medium. Transition Metal Chemistry. 2008;33(3):3111‒3316.
- Huie RE, Peterson NC. Trace Atmospheric Constituents; Properties, Transformations & Fates. In: Schwartz SE (Eds.), John Wiley and Sons, New York, USA. 1983. p.118‒143.
- Hoffmann MR, Boyce SD. Trace Atmospheric Constituents: Properties, Transformations and Fates. In: Schwartz SE (Eds.), John Wiley and Sons, New York, USA. 1983. p. 147‒189.
- Hoffman MR, Jacob DJ. SO2, NO and NO2 Oxidation Mechanisms. In: Calvert JG (Eds.), Atmospheric Considerations, Butterworth, Boston, USA. 1984. p.101‒172.
- Herrmann H. Kinetics of aqueous phase reactions relevant to atmospheric chemistry. Chemical Reviews. 2003;103(12):4691‒4716.
- Huie RE, Wayne Sieck L. S-Centered Radicals. In: Alfassi ZB (Eds.), John Wiley & Sons, New York, USA. 1999. p.63‒99.
- Prasad DSN, Rani A, Gupta KS. Surface-Catalyzed oxidation of Sulfur(IV) in Aqueous silica and copper(II) oxide suspensions. Environmental Science and Technology. 1992;26(7):1361‒1368.
- Huss A, Lim PK, Eckert CA. On the ‘uncatalyzed’ oxidation of sulfur(IV) in aqueous solutions. Journal of American Chemistry Society. 1978;100(19):6252‒6253.
- Huss A, Lim PK, Eckert CA. Oxidation of aqueous sulfur dioxide. 1. Homogenous manganese (II) and iron (II) catalysis at low pH. Journal of Physical Chemistry. 1982; 86(21):4224‒4228.
- Huss A, Lim PK, Eckert CA. Oxidation of sulfur dioxide. 2. High pressure studies and proposed reaction mechanisms. Journal of Physical Chemistry. 1982;86(21):4229‒4233.
- Huss A, Lim PK, Eckert CA. Oxidation of sulfur dioxide. 3. The effects of chelating agents and phenolic antioxidants. Journal of Physical Chemistry. 1982;86(21):4233‒4237.
- Huie RE, Wayne Sieck L. S-Centered Radicals. In: Alfassi Z B (Eds.), John Wiley & Sons, New York, USA. 1999. p.63‒99. 10
- Huie RE, Neta P. Chemical behaviour of SO3- and SO5- radicals in aqueous solutions. Journal of Physical Chemistry. 1984;88(23):5665‒5669.
- Bigelow SL. Catalytic effects in the oxidation of sodium sulfite by air oxygen. Zeitschriftfuer Physikalische Chemie. 1988;26:493‒532.
- Alyea HN, Backstrom HLJ. The inhibitive action of alcohols on the autoxidation of sodium sulfite. Journal of American Chemical Society. 1929;51(1):90‒107.
- Altwicker ER. Advances in Environmental Sciences and Engineering. In: Pfaffin JR & Zieglar EN (Eds.), Gordon and Breach Science Publishers, New York, USA. 1980. p. 80‒91.
- Lee YJ, Rochelle GT. Oxidative degradation of organic acid conjugated with sulfite oxidation in flue gas desulfurization: products, kinetics and mechanism. Environ Sci Technol. 1987;21(3):266‒271.
- Grgic I, Dovzan A, Bercic G, et al. The effect of atmospheric organic compounds on the Fecatalyzed S(IV) autoxidation in aqueous solution. Journal of Atmospheric Chemistry. 1998;29(3):315‒337.
- Wolf A, Deutsch F, Hoffmann P, et al. The influence of oxalate on Fe-catalyzed S(IV) oxidation by oxygen in aqueous solutions. Journal of Atmospheric Chemistry. 2000;37(2):125‒135.
- Ziajka J, Beer F, Warneck P. Iron-catalyzed oxidation of bisulfite aqueous solution: Evidence for a free radical chain mechanism. Atmospheric Environment. 1994;28(15):2549‒2552.
- Pasuik BW, Bronikowski T, Ulejczyk M. Solubilization of organics in water coupled with sulfite Autoxidation. Water Research. 1997;31(7):1767‒1775.
- Pasuik BW, Bronikowski T, Ulejczyk M. Chemical intractions of aqueous phase pollutants: Sulfur dioxide and sobrerol. In: Schurath U & Roselieb R (Eds.), Aachen, Germany. 1998. p.1‒4.
- Pasuik BW, Bronikowski T, Ulejczyk M. Oscillations in the rate of S(IV) autoxidation inhibited by sobrerol. In: Vogt R & Axelsdottir G (Eds.), Aachen, Germany. 1999. p.195‒198.
- Pasuik BW, Bronikowski T, Ulejczyk M. Transformation of atmospheric constituents and pollutants induced by S(IV) Autoxidation-Chemistry and kinetics. Chemical Mechanism Development. 2000. p.123‒126.
- Sameena B, Faiyaz H, Prasad DSN. Kinetics of formic acid inhibited uncatalysed and Co2O3 catalysed autoxidation of S(IV) in alkaline medium. Pelagia research library. 2013; 4(1):122‒131.
- Irena W, Anna M. Mn(II) catalysed and its inhibition by acetic acid in aqueous solutions. Journal of Atmospheric Chemistry. 2008;60(1):1‒17.
- Arun KS, Parashar P, Gupta AK, et al. Formic Acid inhibited Ag (I) Catalysed Autoxidation of S(IV) in Acidic Medium. Journal of Chemistry and Chemical Sciences. 2015; 5(12):760‒777.
- Arun KS, Parashar P, Gupta AK, et al. Ag(I) catalysed autoxidation of S(IV) and its inhibition by Iso propyl alcohol in acidic medium. Chemical Sciences Review and Letters.2016;17(5):14‒23.
- Arun KS, Rashmi S, Prasad DSN. Kinetics and mechanism of uncatalysed and Ag (I) catalysed autoxidation of S(IV) and its inhibition by Iso amyl alcohol in acidic aqueous solutions. International Journal of Modern Sciences and Engineering Technology. 2015;2(12):31‒40.
- Yogpal D, Gupta KS. Role of some organic organicss on oxidation of SO2 in rain water medium. Environ Sci Pollut Res Int. 2014;21(5):3474 ‒3483.
- Yogpal D, Gupta KS. The influence of hydroxyl organic compounds on the oxidation of SO2 by oxygen. Environ Sci Pollut Res Int. 2014;21(13):7808‒7817.
- Podkrajsek B, Grgic I, Tursic J, et al. Influence of atmospheric carboxylic acids on catalytic oxidation of sulfur(IV). Journal of Atmospheric Chemistry. 2006;54(2):103‒120.
- Gupta KS, Mehta RK, Sharma AK, et al. Kinetics of uninhibited and ethanol inhibited CoO, Co2O3 catalysed autoxidation of S(IV) in alkaline medium. Transition Metal Chemistry. 2008;33:809‒817.
- Connick RE, Zhang YX, Lee S, et al. kinetics and mechanism of the oxidation of HSO3 by O2, the uncatalyzed reaction. Inorganic Chemistry. 1995;34(18):4543‒4553.

©2017 Sharma, et al. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.







