Journal of
eISSN: 2473-0831


Research Article Volume 3 Issue 8
Correspondence: Lamia M Abd El Halim, National Organization for Drug Control and Research (NODCAR), 6 Abu Hazem Street, Pyramids Ave, P.O. Box 29, Giza, Egypt
Received: November 03, 2016 | Published: December 30, 2016
Citation: Ramadan NK, Halim LMAE, Sanabary HFE, Salem MY (2016) Thermoanalytical Study and Purity Determination of Trospium Chloride and Tiemonium Methylsulphate. J Anal Pharm Res 3(8): 00086. DOI: 10.15406/japlr.2016.03.00086
The thermal behavior of quaternary ammonium compounds; Trospium chloride and Tiemonium methylsulphate was investigated using different thermal techniques. The thermogravimetry method was used to study the thermal degradation and kinetic parameters; activation energy (Ea), frequency factor (A), and reaction order (n) of both drugs. The data revealed that the cited drugs followed first order kinetic behavior. The fragmentation pathway of both drugs with mass spectrometry was taken as example; to correlate the thermal decomposition with the resulted MS-EI. The melting point and purity were determined using DSC and Van’t Hoff equation for the studied drugs. The results were in agreement with the recommended pharmacopoeias.
Keywords: trospium chloride, tiemonium methylsulphate, thermal analysis, van’t hoff, ammonium, thermogravimetry, fragmentation
TGA, thermogravimetric analysis; DTG, derivative thermogravimetric analysis; DTA, differential thermal analysis; DSC, differential scanning calorimetry; PD, purity determination; TG, thermogravimetry; Ea, activation energy; MS-EI, mass spectrometry electron impact
Trospium chloride {spiro 8-azoniabicyclo3,2,1 octane-8,1-pyrrolidinium]-3 [(hydroxydiphenyl- acetyl)-oxychloride] (1α, 3β, 5α)} is an antimuscarinic agent indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency.1 Tiemonium methylsulphate{4-[3-Hydroxy-3-phenyl-3-(2-thienyl) propyl]-4-methyl-morpholinium methyl sulphate} is used as antimuscarinic with peripheral effects similar to those of atropine and is used in the relief of visceral spasms. It has been used as an antispasmodic.1
Determination of Trospium chloride is described in European pharmacopoeia by titrimetric method;2 using 0.1 M silver nitrate as titrant, then the end point was determined potentiometrically. The other methds are a fluorimetric method after derivatization with benoxaprofen chloride,3 LC–MS method,4 RP-UFLC5 and Stability chromatographic methods.6 Few methods were reported for analysis of Tiemonium methylsulphate; Swift Quantification of Fenofibrate and Tiemonium methylsulfate by using Particle Induced X-Ray Emission,7 UV-spectroscopic method.8 Stability Indicating Chromatographic methods9 and Stability indicating spectrophotometric methods.10
Thermal analysis techniques cover all methods in which a physical property is monitored as a function of temperature or time, whilst the sample is being heated or cooled under controlled conditions. Thermogravimetry (TG) and differential scanning calorimetry (DSC) are useful techniques that have been successfully applied in the pharmaceutical industry to reveal important information regarding, the physicochemical properties of drug and excipient molecules such as polymorphism, stability, purity, formulation compatibility among others, and assessing the drug degradation kinetics. There are definitive advantages to employing multiple thermal analysis methods to attain varying views of the physicochemical properties of pharmaceuticals. The determination of the key physical and chemical properties of a new material is essential.11‒15 Therefore, the aim of this study was to evaluate the thermal characterization of Trospium chloride and Tiemonium methylsulphate using a variety of techniques including TGA/DTG, DTA and DSC. The search of thermal degradation and kinetics, were carried out to help understanding the solid-state characterization, evaluate the quality control and stability for these important active pharmaceutical ingredients.
The determination of purity by DSC of the used compounds in thermal analysis is very important as the chromatographic methods are used to determine purity of the used compounds in comparison with a standard samples. On the other hand, DSC technique can be used for the determination of purity based on the assumption that the impurities will lower the melting point of a pure substance. The melting transition of pure (100% crystalline substance) should be infinitely sharp, but impurities or defects in the crystal structure will broaden the melting range and lower the melting point.
As well DSC technique provides unique information in relation to thermodynamic data of the system studies including characterization, polymorphism identification, and purity evaluation of drugs, compatibility studies for the pharmaceutical formulation that the chromatographic systems don’t provide.
Instruments
TG/DTG and DTA curves of drug substances: were recorded using Simultaneous Shimadzu thermogravimetric analyzer TGA-60 H (Tokyo – Japan), with TA 60 software in dry nitrogen atmosphere at a flow rate of 30ml/ min in platinum crucible containing aluminum oxide as a reference. The experiments were performed from ambient temperature up to 600 ºC with a heating rate of 10ºC/min. The sample mass was about 5mg of the drug without any further treatment. The kinetic parameters of decomposition such as, activation energy (Ea) and frequency factor (A), and reaction order (n) were calculated from TGA/ DTG curves. The mathematical models of Arrhenius,16 Horowitz Metzger17 and Coats, Redfern18 were used for kinetic parameters determination.
The DSC curves: were recorded using Shimadzu-DSC 50 (Tokyo – Japan), in dynamic nitrogen atmosphere with a constant flow of 30ml/min, and heating rate of 10ºC/min, up to temperature 300ºC. The sample with a mass of about 1.80mg was packed in platinum pan. DSC equipment was preliminarily calibrated with standard reference of indium. The purity determination was performed using heating rate of 10ºC/min in the temperature range from 25 to 400ºC in nitrogen atmosphere.
Mass spectrometry electron impact (MS-EI): Mass spectra of Trospium chloride and Tiemonium methylsulphate were recorded using Shimadzu-GC-MS-QP 1000 EX quadruple mass spectrometer with Electron Impact detector equipped with GC-MS data system.
Melting point: Opti Melt Automated Melting Point System, SRS Stanford Research System.
Trospium chloride-Pure sample was kindly supplied by Hekma Pharma, Egypt, B.N. 21787. Its purity was found to be 99.95±0.644% according to the official method. Trospikan tablet was supplied by Hikma Pharma, (Cairo, Egypt), B.N.003. Each tablet is claimed to contain 20mg of Trospium chloride. Tiemonium methylsulphate-Pure sample was kindly supplied by Centaur Pharmaceuticals PVT. LTD. India, B.N. 20094607. Its purity was found to be 99.73%±0.504 according to the manufacturer's method. Spasmofree tablet was supplied by Adwia Pharma, (Cairo, Egypt), B.N. 031013. Each tablet is claimed to contain 50.0mg of Tiemonium methylsulphate.
Thermal characterization
For Trospium chloride: The TGA/DTG curves of Trospium chloride presented in (Figure 1) show that the drug is thermally decomposed in two steps. The first step occurs in the temperature range of 221.26-349.84ºC with the loss of 60.2645% which may be due to the
C12H16NO3Cl molecule. The second step occurs at 373.22-428.13ºC with about 39.305% weight loss which may be attributed to the loss of C13H14 molecule. The suggested thermal decomposition pathway of Trospium chloride is summarized in (Scheme 1).
The DTA curve (Figure 2) exhibits endothermic peaks. The first sharp endothermic peak at 267.51°C is due to the melting of the compound. The second sharp endothermic peak at 303.53°C is attributed to the first decomposition corresponding to the first mass loss while the third broad endothermic peak at 420.12°C is attributed to the second mass loss as observed in TGA/DTG thermogram curves shown in (Figure 1). The broad exothermic peaks at 553.24°C are due to the pyrolysis of the compound.
For Tiemonium methylsulphate: The TGA/DTG curves of Tiemonium methylsulphate presented in (Figure 3) show that the drug is thermally decomposed in three steps. The first step occurs in the temperature range of 71.95-157.12ºC with the loss of 10.78% which may be due to the loss of C2H6O molecule. The second step occurs at 242.30-347.98ºC with about 37.534% weight loss which may be attributed to the loss of C4H4S2O3 The third step occurs in the temperature range 523.76-605.43ºC which may be due to the loss of C13H18NO2. The suggested thermal decomposition pathway of Tiemonium methylsulphate is summarized in (Scheme 2).
The DTA curve (Figure 4) exhibits endothermic peaks. The first endothermic peak at 143.08 °C is due to the melting of the compound and the first step of decomposition. The second endothermic peak at 285.03°C is attributed to the second decomposition corresponding to the second mass loss while the third broad exothermic peak at 577.52°C is attributed to the third mass loss as observed in TGA/DTG thermogram curves shown in (Figure 3).
Kinetic analysis
The kinetic studies of the main thermal decomposition (degradation) steps of Trospium chloride and Tiemonium methylsulphate were investigated by mathematical models (1, 2 and 3) of Arrhenius Equation Method (Figure 5 & 6), Horowitz and Metzger (HM) (Figure 7 & 8) and Coats-Redfern (CR) (Figure 9 & 10) respectively. The kinetic parameters obtained for both drugs are summarized in (Table 1).

Figure 5 Kinetic plot of LnK versus 1/T for Trospium chloride using mathematical model of Arrhenius.
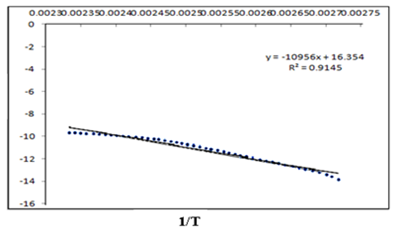
Figure 6 Kinetic plot of LnK versus 1/T for Tiemonium methylsulphate using mathematical model of Arrhenius.
|
Kinetic |
Arrhenius |
Horowitz Metzger(HM) |
Coats- Redfern (CR) |
||||
|
Equation |
|
|
|||||
|
Kinetic |
|||||||
|
parameter |
|||||||
|
Ea kJ/mol |
180.688 (Trospium) 90.088 (Tiemonium) |
149.632 (Trospium) |
162.172 (Trospium) 97.316 (Tiemonium) |
||||
|
80.573 (Tiemonium) |
|||||||
|
A (S-1) |
1.32 x 1015 (Trospium) 1.27 x 108 (Tiemonium) |
1.84 x 1014 (Trospium) |
2.08 x 1014 (Trospium) 2.64 x 109 (Tiemonium) |
||||
|
6.91 x 109 (Tiemonium) |
|||||||
|
ΔS* (kJ/mol) |
39.622 (Trospium) |
23.241(Trospium) |
24.246 (Trospium) |
||||
|
-92.579 (Tiemonium) |
-59.349 (Tiemonium) |
-67.358 (Tiemonium) |
|||||
|
ΔH* (kJ/mol) |
176.198 (Trospium) 86.621 (Tiemonium) |
145.143 (Trospium) |
157.679 (Trospium) 93.847 (Tiemonium) |
||||
|
77.106 (Tiemonium) |
|||||||
|
ΔG* (kJ/mol) |
154.802 (Trospium) 125.226 (Tiemonium) |
132.593 (Trospium) |
144.586 (Trospium) 121.935 (Tiemonium) |
||||
|
101.855 (Tiemonium) |
|||||||
|
n (For both drugs) |
1 |
1 |
1 |
||||
|
r |
0.9685 (Trospium) 0.9563 (Tiemonium) |
0.9614 (Trospium) |
0.9632 (Trospium) 0.9882 (Tiemonium) |
||||
|
|
|
0.9555 (Tiemonium) |
|||||
Table 1 Thermo analytical data for Trospium chloride and Tiemonium methylsulphate using Arrhenius equation, Horowitz-Metzger (HM) and Coats-Redfern (CR)
(1)
Where, (A) is the pre- exponential term, E is the activation energy, R = gas constant, T = temperature in degrees Kelvin.
(2)
Where, Wf was the mass loss at the completion of the decomposition reaction, W was the mass loss up to temperature T, R was the gas constant, Ts was the DTG peak temperature and θ= T-Ts. A plot of log [log Wf / (Wf - W)] against θ would give a straight line and E* could be calculated from the slope.
(3)
Where, Φ was the heating rate. Since 1- 2RT / E* ≈1, the plot of the left-hand side of equation against 1/T would give a straight line. E* was then calculated from the slope and the Arrhenius constant (A) was obtained from the intercept.
Correlation between the mass spectra and thermal behaviour
For Trospium chloride: The mass spectra of Trospium chloride are presented graphically in (Figure 11). Trospium chloride decompose give fragments, with m/z = 224 and the second m/z = 170. The other molecular ions peaks appeared in the mass spectra are attributed to the fragmentation of the drug. The m/z = 224 represent C12H16NO3, while m/z = 170 represent C13H14. The results were presented in (Scheme 1).
For Tiemonium methylsulphate: The mass spectra of Tiemonium methylsulphate are presented graphically in (Figure 12). Tiemonium methylsulphate decompose give fragments, with m/z = 383 and the second m/z = 224. The other molecular ions peaks appeared in the mass spectra are attributed to the fragmentation of the drug. The m/z = 383 represent C17H21NO5S2, while m/z = 224 represent C13H17NO2. The results were presented in (Scheme 2).
Application of differential scanning calorimetry for purity determination
The melting transitions of a pure 100% crystalline material should be infinitely sharp, but impurities or defects in the crystal structure will broaden the melting range and lower the final melting point to a temperature lower than to [19]. The effect of impurities on To of Trospium chloride and Tiemonium methylsulphate was determined by DSC method based on Van't Hoff equation.
Where, Tѕ is the sample peak at temperature (K), Tο is the melting point of pure component (Kalvin), R is the gas constant, X is the concentration of impurity (grams fraction), ∆Hf = Heat of fusion of pure component (J mg -1), and F is the fraction of sample melted at Ts.
The melting points obtained from DSC curves of Trospium chloride and Tiemonium methylsulphate (Figure 13 & 14) were in accordance with those of officially reported (Table 2), justifying the use of DSC as a routine technique for identification of drugs through the melting point 20-22.
|
Name of Drug |
Degree of Purity% |
Melting Point ºC |
|
|
||||
|
Trospium Chloride |
DSC |
Official method2* |
DTA |
DSC |
Melting point apparatus |
Reported method20 |
||
|
99.99 |
99.95 |
267.51 |
266.12 |
268 |
266 - 268 |
|||
|
Tiemonium methylsulphate |
DSC |
Manufacturer’s method21** |
DTA |
DSC |
Melting point apparatus |
Manufacturer’s method22 |
||
|
|
99.99 |
99.73 |
143.08 |
144.75 |
143.8 |
144.32 |
||
Table 2 Comparison between the degree of purity of Trospium chloride and Tiemonium methylsulphate in pure form obtained by DSC and official method and between melting point obtained by DTA, DSC and those obtained by melting point apparatus and reported method
The thermal stability of Trospium chloride and Tiemonium methylsulphate using different thermal techniques (TGA/DTG, DTA, and DSC), was studied. The kinetic studies of Trospium chloride and Tiemonium methylsulphate showed a thermal behavior characteristic to first order according to Ea. The correlation between mass spectra and thermal behavior of both drugs was studied. The data revealed correlation between the three techniques. DSC provides a rapid method for purity determination attending a value between 98.5-101.5 %, which is in agreement with the official pharmacopoeia.
None.
None.

©2016 Ramadan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.