Journal of
eISSN: 2473-0831


Research Article Volume 3 Issue 3
Correspondence: Narotam Sharma, Central Molecular Research Laboratory, Department of Biochemistry, Shri Guru Ram Rai Institute of Medical & Health Sciences, Patel Nagar, Dehradun-248001, Uttarakhand, India
Received: October 17, 2016 | Published: October 24, 2016
Citation: Chaurasia A, Sangela V, Jawed B, Sehgal M, Prashar V, et al. (2016) Characterization of Hepatitis C Virus (HCV) Genotypes with Spectrum of its Viral Load. J Anal Pharm Res 3(3): 00058. DOI: 10.15406/japlr.2016.03.00058
The Hepatitis C virus (HCV) is the most common cause of chronic liver disease and is increasing worldwide. Proposed study includes 24 clinical samples, processed for HCV RNA viral load and HCV genotyping. HCV RNA viral load ranges in between 1.00×101 IU/ml to > 1.00×108 IU/ml and the cases with high viral load were further processed for the genotyping. Out of 24 specimens characterized genotype 3a was prevalent in 72.72% (8/24) cases and HCV genotype 1b was in 27.27% (3/24) cases followed by HCV genotype 4a, which was in 27.27% (3/24) cases. In 13 cases target was not detected due to low viral load. 8 cases were for HCV genotype 3a of which 5 were males & 3 were females, followed by HCV genotype 1b (3 males) & 4a (3 females). The genotype 3a was found in 8 (72.72%) patients. Genotype 1b was seen in 3 (27.27%) patients. Genotype 4a was seen in 3 (27.27%) patients. Genotype 2a was seen in only 1 (9.09%) patient. From the current study genotype 3a was most prevalent and can be further studied. The outcome of HCV genotyping is of almost clinical value as there are various regimens available to treat different types of HCV genotypes.
Keywords: genotype; sexual transmission; cirrhosis; real time PCR; replicating viruses; chronic
Hepatitis C virus (HCV) is an enveloped single-stranded RNA virus which appears to be distantly related to flaviviruses, although HCV is not transmitted by arthropod vectors.1,2 Hepatitis C virus is associated with chronic liver disease and also with primary liver cancer in many countries. HCV is mostly transmitted through exposure to infective blood.3 This may happen through transfusions of HCV-contaminated blood and blood products, contaminated injections during medical procedures, and through injection drug use.4–6 exual transmission is also possible, but is much less common. Unless successfully treated with medication, chronic Hepatitis C infection can cause other serious health problems, such as cirrhosis, liver cancer and liver failure.7–10 Hepatitis C is divided into six distinct genotypes throughout the world with multiple subtypes in each genotype class. In the current project the patient with HCV infection were consider & further quantification of 5’untranslated region of the HCV genome & its genotypic characterization were determined in spectrum of its viral load.11–15
A total of 24 clinical samples were considered in this study. Blood samples were taken from the infected cases of HCV and further processed at Central Molecular Research Laboratory (CMRL), Shri Guru Ram Rai Institute of Medical & Health science (SGRRIM & HS), Patel Nagar, Dehradun (U.K.) for the quantification of HCV & identification of HCV genotypes. Pre-processing of the sample collection: 5 ml EDTA Whole blood was centrifuged at 5,000 rpm for 10-15 minutes. Isolated serum was used further for RNA isolation. RNA was isolated by High Pure Viral Nucleic Acid Extraction kit. The specimens were processed for HCV viral load by Roche COBAS TaqMan 48 analyzer & the 5’untranslated region of the HCV genome was amplified for the quantification of HCV virus.16,17 Further HCV genotypic characterization was achieved by nested PCR targeting core region with genotype specific PCR primers.18,19
A total of 24 clinical samples were collected from different Departments of Shri Mahant Indiresh Hospital, Patel Nagar, Dehradun U.K., India which includes medicine, surgery, pediatric, gastro. Etc was included for the proposed study. It was seen that viral load of HCV RNA ranges in between 1.00×101 IU/ml to > 1.00×108 IU/ml. Sample having the high viral load were further processed for the genotyping & those less than 2.0×103 IU/ml were not considered because the sensitivity is low of the assay. The nested PCR yielded different amplicons for different HCV genotypes (Figure 1–4). Out of 24 specimens characterized genotype 3a was prevalent in 72.72% (8/24) cases and HCV genotype 1b was in 27.27% (3/24) cases followed by HCV genotype 4a, which was in 27.27% (3/24) cases. In 13 cases target was not detected due to low viral load (limit of detection of the protocol is 25 IU/ml of HCV RNA) & all these 13 cases were not considered for further genotyping. It was also observed that in viral load ranging in between 1.00×104 to 1.00×107 IU/ml maximum number of cases were 10 and in the same spectrum HCV genotype 1b,2a,3a,4a were characterized as tabulated in Table 1-3. Out of 24 samples different age groups were also considered which includes from 0-20 years, 21-40 years, 41-60 years, & above 60 years, & the number of cases in these particular age groups were 1 (Female), 8 (7 Male & 1 Female) , 14 (Males), 1 (Female) respectively.
|
Sl.No. |
HCV RNA Titer (IU/ml) |
No. of Cases |
HCV Genotypes |
1a |
1b |
2a |
2b |
3a |
3b |
4a |
5a |
|
1 |
Target not detected |
13 |
|||||||||
|
2 |
1.00×101 – 1.00×103 |
1 |
1b,3a,4a |
- |
1 |
- |
- |
1 |
- |
1 |
- |
|
3 |
1.00×104- 1.00×107 |
10 |
1b,2a,3a,4a |
- |
3 |
1 |
- |
8 |
- |
3 |
- |
|
4 |
>1.00×108 |
0 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
|
Total Cases = |
24 |
||||||||||
Table 1 Spectrum of HCV RNA viral load & its genotypes characterization
|
Age Group in Years |
No. of Cases |
HCV Genotypes |
|
0-20 |
1 |
3a |
|
21-40 |
8 |
1b, 2a, 3a, 4a |
|
41-60 |
14 |
1b, 3a, 4a |
|
Above 60 |
1 |
Genotype not detected due to low viral load |
Table 2 Age wise HCV viral load and genotype distribution pattern
|
HCV Genotype Detected |
No. of Cases |
Percentage |
|
1a |
- |
- |
|
1b |
3 |
27.27% |
|
2a |
1 |
9.09% |
|
2b |
- |
- |
|
3a |
8 |
72.72% |
|
3b |
- |
- |
|
4a |
3 |
27.27% |
Table 3 Percentage wise distribution of HCV genotypes
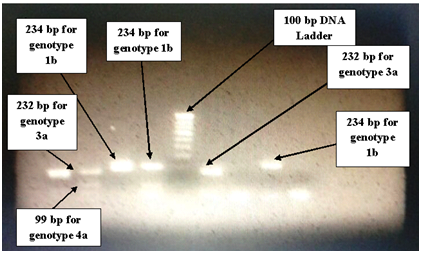
Figure 1 Gel picture showing different genotypes of HCV at different base pairs.
Well No.1: HCV genotype 3a (232 bp).
Well No.2: HCV genotype 3a (232 bp), HCV genotype 4a (99 bp).
Well No.3: HCV genotype 1b (234 bp).
Well No.4: HCV genotype 1b (234 bp), HCV genotype 4a (99 bp).
Well No.5: 100 bp DNA Ladder.
Well No.6: HCV genotype 3a (232 bp).
Well No.7: Negative (Genotype not detected due to low viral load).
Well No.8: HCV genotype 1b (234 bp).
Differences among HCV genotypes in geographic distributions have provided investigators with an epidemiological marker that can be used to find the source of HCV infection in a given population & for further prognosis [20,21]. The current study includes the collection of 24 specimens which were confirmed positive for HCV by serological findings. As the hospital includes the involvement of patients from hilly areas of Uttarakhand & Himachal Pradesh along with some parts of West U.P. Out of 11 samples which was processed for genotyping, 8 cases were for HCV genotype 3a of which 5 were males & 3 females, followed by HCV genotype 1b (3 males) & 4a (3 males). The genotype 3a was observed in 8 (72.72%) patients. Genotype 1b was seen in 3 (27.27%) patients. Genotype 4a was seen in 3 (27.27%) patients. Genotype 2a was seen in only 1 (9.09%) patient. Our findings include the prevalence of HCV genotype 3a in the clinical isolates, although HCV genotypes 1, 2 and 3 were also detected. From the current study genotype 3a was most prevalent and was present in males (the reason is not clear but it may be due to injection, drug abuse, needle stick accidents and transfusion of blood, intravenous drug users & tattooing etc). Similar study was also done by Pushpalatha, Chakravarti, Anita, Gaurav, Dogra et al.20–25 This work can be further evaluated to study the exact HCV genotyping characterization in the Northern Indian population with more number of samples because this study was limited to the 24 samples only.
The outcome of HCV genotyping is of almost clinical value as there are various regimens available to treat different types of HCV genotypes like Simeprevir, Sofosbuvir etc for genotype 1. Sofosbuvir/R for genotype 2 & Sofosbuvir/R for genotype 3. There are various alternative therapies also available to treat Hepatitis C infection like Milk Thistle, Green tea extract, Glycyrrhizin but currently there is no vaccine available to prevent the Hepatitis C infection. The distribution of HCV genotypes vary according to the geographical region.26–28 The HCV genotype 3a is one of the most replicating viruses known to damage hepatic cells & thus requires proper line of treatment thoroughly during the diagnosis. Further study can be done with more number of clinical isolates to get the exact epidemiological profiling of HCV genotypes.
The authors are grateful to Honorable Chairman, Shri Guru Ram Rai Education Mission for his kind support and guidance.
The authors declare no conflict of interest.
None.

©2016 Chaurasia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.