Journal of
eISSN: 2473-0831


Research Article Volume 2 Issue 4
Correspondence: Nachiket S Dighe, Associate Professor & HOD, Department of Pharmaceutical Chemistry, Pravara Rural College of Pharmacy, Pravaranagar, A/P- Loni Bk, Taluka-Rahata, Dist-Ahmednagar 413736, India, Tel 9890215729
Received: May 18, 2016 | Published: June 3, 2016
Citation: Dighe NS, Tambe DL, Dighe AS, Musmade DS, Nirmal SA (2016) Design, Synthesis and Anti-Depressant Activity of Some Novel Coumarin Derivatives. J Anal Pharm Res 2(4): 00026. DOI: 10.15406/japlr.2016.02.00026
The present research work deals with the synthesis, characterization and to evaluate the synthesized compound for antidepressant activity of a series of coumarin derivatives. Basic coumarin is prepared by Perkin reaction, which further reacted with aryl thiourea by cyclization reaction leads to produce 3-(2-(phenylaminothiazol-4-yl)-2H-chromen-2-one. Totally twelve compounds, were synthesized by conventional method and their purity was determined by TLC and they were characterized by IR and NMR spectroscopic methods. Antidepressant activity of all the synthesized compounds was evaluated by despair swim test by using Sprague Dawley Rats. Standard drug Imipramine was used as the control. In the despair swim test, all the synthesized derivatives showed antidepressant activity. Among them three Compounds (A4, A5 and A9) showed significant antidepressant activity comparing with control drug imipramine and some compound shows mild antidepressant activity. These results are useful for the further investigation in the future.
Keywords: antidepressant activity, coumarin, despair swims test, perkin reaction, sprague dawley rat
Depression is a common mental disorder, characterized by sadness, loss of interest or pleasure, feelings of guilt or low self-worth, disturbed sleep or appetite, feelings of tiredness and poor concentration. Depressive episode involves symptoms such as depressed mood, loss of interest and enjoyment, and increased fatigability. Depression is a major psychiatric disorder affecting nearly 21% of the world population and imposes a substantial health burden on society.1,2 Depression is a significant contributor to the global burden of disease and affects people in all communities across the world. Today, depression is estimated to affect 350 million people. The World Mental Health Survey conducted in 17 countries found that on average about 1 in 20 people reported having an episode of depression in the previous year. Antidepressants are the drugs used to treat depression thereby elevates mood and modifies the behavior. The discovery of antidepressants could be described as a ‘lucky accident’. During the 1950s, while carrying out trials on a new medication for tuberculosis (TB), researchers noticed that the medication also had a mood improving effect. Half a century ago, antidepressants were discovered by serendipity. There is much more area in which the research and development for antidepressant drugs is done. But till the glob required the development of new antidepressants which have greater efficacy, absence of side effects, lack of toxicity in over dose and earlier onset of action.3
The forced swim test is a rodent behavioral test used for evaluation of antidepressant drugs, antidepressant efficacy of new compounds, and experimental manipulations that are aimed at rendering or preventing depressive-like states. Decrease in the duration of immobility which denotes the antidepressant activity majorly.4
There are many coumarin and its analogs are very important chemical synthons of pharmacological acceptations and pharmaceutical use. They possess a variety of biological activities including antibacterial,5 anti-clotting and anti-thrombotic, anti-inflammatory, anti-carcinogenic,6 anti-HIV activities, hepatoprotective, antidepressant and antiplatelet aggregation activities.7 Coumarin shows antidepressant activity by various mechanisms such as MAO inhibitors, triple reuptake inhibitor.8 But there are much further investigation in future may be done for coumarin as antidepressant.
Chemistry
Loba Chemie is a manufacturer of Laboratory Reagents and Fine Chemicals for industrial use. Monitoration of reactions is done by TLC using silica gel G. The melting point was determined by using Kjeldahl flask containing liquid paraffin. FTIR-spectrophotometer (JASCO) is used for IR spectra recording using KBr pellets. BRUKER AVANCE II 400 NMR spectrometer is used for 1h NMR spectral determination in DMSO using tetra methyl silane (TMS) as internal reference.
Procedure for scheme
Synthesis of 3-Acetyl-2H-Chromen-2-one: A mixture of salicyaldehyde (0.5moL) and ethyl acetoacetate (0.5moL) was stirred and cooled. To this mixture 0.5-1ml of piperidine was added with shaking. The mixture was maintained at freezing temperature for 30-45 minutes resulting in a yellow colored solid mass, which was separated out. It was recrystallized from ethanol to get the target compound (I).
Synthesis of 3-(Bromoacetyl)-2H-Chromen-2-one: To a solution of compound (I) (0.25moL) in 200 ml of alcohol free chloroform, bromine (0.25ml) in 25mL of chloroform was added with intermittent shaking. The mixture was warmed to decompose an addition product. The mixture was heated for 20min, cooled and filtered to get a solid mass which on washing with ether gave the desired product. It was recrystallized from acetic acid to give colorless needles (II).
Synthesis of 3-(2-Arylamino-1, 3-Thiazol-4-yl)-2- Chromen-one [A1-A12]: A suspension of compound (II) (0.1moL) in 175mL of hot ethanol was heated with arylthiourea (0.1moL), giving a clear solution that soon deposited as crystals. It was filtered, washed with ethanol and then boiled with water containing sodium acetate which yielded the target compound. The product obtained was recrystallized with ethanol (III) (Figure 1).
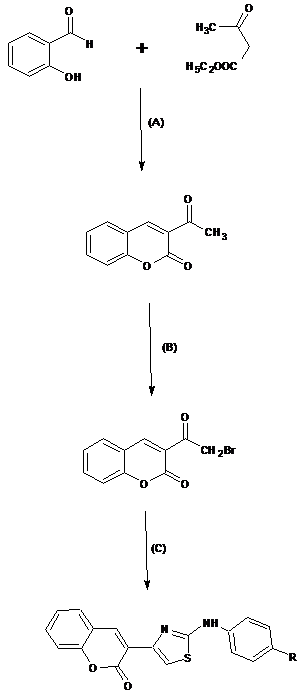
Figure 1 Reagents and conditions; (a) Piperidine, Ethanol (b) Br2, CHCl3, Heat (c) Substituted arylthiourea, Heat.
Pharmacology
Rat-Sprague Dawley having weight 220-255gm and are 8-12 weeks old, was obtained from National Institute of Bioscience, Pune. They were housed in autoclaved polypropylene cages in groups of 2-3 rats per cage and kept in a room maintained at 19 to 25°C and humidity 45 to 65 % with a 12-h light/dark cycle. They were allowed to acclimatize for four days before the experiments and were given free access to Standard sterilized extruded rodent diet was provided ad libitum, Reverse Osmosis water treated with UV light was provided ad libitum in autoclaved polypropylene bottles and Autoclaved corn cob was used as bedding material.
Standard operating procedures of the Prado Pvt. Ltd. are referred as present study with the guidelines provided by the CPCSEA was published in The Gazette of India, December 15, 1998. Prior approval of the Institutional Animal Ethics Committee (IAEC) was obtained before initiation of the study (IAEC-13-002).
Antidepressant activity (forced swim test in rat)
Porsolt et al.,9 is a founder of Behavioral despair or forced swim test (FST) as a model to test antidepressant activity.9,10 It was recommended that mice when forced to swim in blocked space from where they cannot bypassing are induced to a unique action of immobility.11 This behavior follows a state of despair which can be reduced by certain agents which are therapeutically competent in human abasement. The behavioral despair test is occupied to assess the antidepressant activity of synthesized compound. Sprague-Dawley rats of 200-27 gm in a faction of two each were used and on the first day of the analysis, (pretest session), rats were personally placed in a circular recipient (Plexiglass cylinder) of dimensions (diameter, 10cm; height, 25cm) containing 10 cm of water 25°C. The animals were left to swim for 6min before being detached, dried and returned to their cages. The experiment was repeated 24 h later, in 5min swim session (test session). The synthesized compounds (25mg, kg-1), and imipramine, as a reference antidepressant drug (25mg, kg-1) were suspended in a 0.5 % aqueous solution of Na CMC (Corboxy Methyl Cellulose). The drugs were given by gavage in a standard volume of 10ml/kg body weight, 1hr prior to the test. Control animals received 0.5% aqueous solution of Na CMC (Corboxy Methyl Cellulose). This test was performed after 1hr, 5hrs and 24hrs of dose administration. For individual animal video recording was made. Then, the rats were dropped individually into the Plexi glass cylinder and left in the water for 6min. After the first 2min of the initial vigorous struggling, the animals were immobile. An immobility time is the time spent by rat floating in water without struggling, making only those moment necessary to keep the head above the water. The total duration of immobility was recorded during the last 5min of the 6min test session.
QSAR methodology
All molecules were drawn in Chem draw ultra 8.0module in Chemoffice 2004 software and imported into TSAR software. Charges were derived using Charge 2-Derive charges option and optimized by using Cosmic-optimize 3D option in the structure menu of the project table. Substituents were defined and descriptors were calculated for whole molecule as well as for the Substituent’s. Several equations were generated correlating both Log (% Immobility) with physicochemical parameters (descriptors) by multiple linear regression analysis (MLR) method. Data was standardized by range and leave one out method was used for cross validation. Models were excluded if correlation was exceeding 0.9 for more rigorous analysis. Correlation matrix was generated to find any Intercorrelation between the descriptors. Intercorrelation between the descriptors in the final equation is less than 0.2 11.
A1: FTIR (KBr Disc cm-1)= 3029(Ar-CH str.), 1700(-C=O str.), 1505(-C=N str), 1235(-C-N str).
1H-NMR(ppm) = 12.00(1H of –COOH); 6.80-7.40(8H of phenyl); 5.0(1H of –NH).
A2: FTIR(KBr Disc cm-1) = 3424(Ar-CH str.), 1702(-C=O str.), 1575(-C=N str), 247(-C-N str).
1H-NMR(ppm) = 6.80-7.40(9H of phenyl); 5.0(1H of-NH).
A3: FTIR(KBr Disc cm-1) = 3452(Ar-CH str.), 1699(-C=O str.), 1576.52(-C=N str), 1273(-C-N str) .
1H-NMR(ppm) = 6.85-7.55(8H of Phenyl); 5.0(1H of –NH).
A4: FTIR(KBr Disc cm-1) = 3504.99(Ar-CH str.), 1735.62(-C=O str.), 1578.45(-C=N str), 1272.79(-C-N str).
1H-NMR(ppm) = 7.00-7.80(8H of phenyl); 5.0(1H of-NH); 4.0(2H of –NH2).
A5: FTIR (KBr Disc cm-1) = 3438.46(Ar-CH str.), 1738.51(-C=O str.), 1589.06(-C=N str), 1278.57(-C-N str).
1H-NMR(ppm) = 6.90-7.60(8H of phenyl); 5.0(1H of –NH).
A6: FTIR(KBr Disc cm-1) = 3516.56(Ar-CH str.), 1743.33(-C=O str.), 1575.56(-C=N str), 1272.79(-C-N str).
1H-NMR(ppm) = 6.80-7.40(8H of phenyl); 5.0(1H of –NH); 4.0(1H of –OH).
A7: FTIR(KBr Disc cm-1) = 3057.58 (Ar-CH str.), 1700.91 (-C=O str.), 1578.45 (-C=N str),1222.65 (-C-N str).
1H-NMR(ppm) = 6.80-7.40(8H of phenyl); 5.0(1H of –NH).
A8: FTIR(KBr Disc cm-1) = 3329.5(Ar-CH str.), 1700.91(-C=O str.), 1552.66(-C=N str), 1376.93(-C-N str).
1H-NMR(ppm) = 6.80-7.40(8H of phenyl); 5.0(1H of –NH).
A9: FTIR (KBr Disc cm-1) = 2945.73(Ar-CH str.), 1701.87(-C=O str.), 1577.49(-C=N str), 1272.79(-C-N str).
1H-NMR(ppm) = 6.80-7.40(8H of phenyl); 5.0(1H of –NH).
A10: FTIR (KBr Disc cm-1) = 3448.10 (Ar-CH str.), 1699.94(-C=O str.), 1590.02(-C=N str), 1271.82(-C-N str).
1H-NMR(ppm) = 6.80-7.40(8H of phenyl); 6.20-6.40(2H of CH=CH); 5.0(2H of –NH); 4.5(2H of NH2).
A11: FTIR(KBr Disc cm-1) = 3306.36(Ar-CH str.), 1810.83(-C=O str.), 1467.56(-C=N str), 1272.79(-C-N str).
1H-NMR(ppm) = 6.80-7.40(8H of phenyl); 5.0(2H of –NH); 4.5(2H of NH2); 0.8-1.4(3H of CH3).
A12: FTIR (KBr Disc cm-1) = 3374.82(Ar-CH str.), 1811.79(-C=O str.), 1500.35(-C=N str), 1271.82(-C-N str).
1H-NMR(ppm) = 6.80-7.40(8H of phenyl); 5.0(2H of –NH); 4.5(2H of NH2).
Antidepressant activity
The test is despair swim test and a Sprague-Dawley rats are used to antidepressant activity study for all the integrate compounds. Standard control Imipramine was used. At the last four minutes of the period the animals show more strong levels of paralysis. With the synthesized compounds three Compounds (A4, A5 and A7) showed powerful antidepressant activity with reference to standard control imipramine (Table 2) (Figure 2). The results were taken as a triplicate and average of each was calculated.
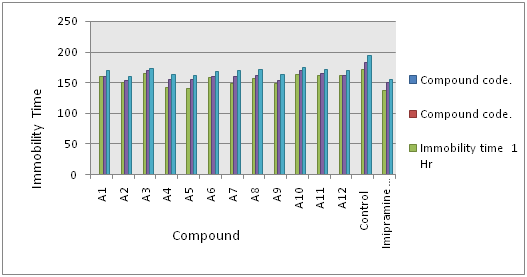
Figure 2 Antidepressant-like effects of Coumarin derivatives test compounds in FST Data are presented as compared to control group. (Compounds code vs Immobility time).
|
Compound Code |
R |
|
A1 |
COOH |
|
A2 |
H |
|
A3 |
NO2 |
|
A4 |
NH2 |
|
A5 |
Cl |
|
A6 |
OH |
|
A7 |
Br |
|
A8 |
NO2 |
|
A9 |
Cl |
|
A10 |
|
|
A11 |
|
|
A12 |
|
Table 1 Synthesis of 3-(2-Arylamino-1, 3-Thiazol-4-yl)-2- Chromen-one [A1-A12].
|
Compound |
Mol. Formula |
Mol. Wt. |
M.P°C |
Yield % |
Elemental analyses Found (Cal.) |
|||
|
|
|
|
|
|
C |
H |
N |
S |
|
A1 |
C19H12N2O4S |
364 |
220-230 |
80 |
62.63(62.15) |
3.29(2.98) |
7.69(7.45) |
8.79(8.47) |
|
A2 |
C18H11N2O2S |
319 |
235-237 |
75 |
67.71(67.38) |
3.44(3.18) |
8.77(8.56) |
10.03(9.78) |
|
A3 |
C18H11N3O4S |
365 |
205-207 |
67 |
59.17(58.86) |
3.01(2.89) |
11.5(11.18) |
8.76(8.49) |
|
A4 |
C18H12N3O2S |
334 |
260-270 |
80 |
64.67(64.38) |
3.59(3.29) |
12.57(12.21) |
9.58(9.23) |
|
A5 |
C18H9Cl2N2O2S |
388 |
210-215 |
78 |
55.67(55.31) |
2.31(1.98) |
7.21(6.85) |
8.24(7.89) |
|
A6 |
C18H11N2O3S |
335 |
265-267 |
69 |
64.47(64.21) |
3.28(2.89) |
8.35(8.04) |
9.55(9.25) |
|
A7 |
C18H10BrN2O2S |
398 |
230-233 |
72 |
54.27(53.98) |
2.51(2.18) |
7.03(6.85) |
8.04(7.85) |
|
A8 |
C18H9N4O6S |
309 |
270-275 |
65 |
69.9(69.57) |
2.91(2.58) |
18.12(17.89) |
10.35(10.08) |
|
A9 |
C18H9Cl2N2O2S |
388 |
190-200 |
71 |
55.67(55.28) |
2.31(1.98) |
7.21(6.98) |
8.24(7.96) |
|
A10 |
C15H11N2O2S |
283 |
234-236 |
69 |
63.6(63.28) |
3.88(3.76) |
9.89(9.69) |
11.3(10.92) |
|
A11 |
C14H9N2O3S |
285 |
240-242 |
62 |
58.94(58.68) |
3.18(2.94) |
9.82(9.68) |
11.22(10.98) |
|
A12 |
C12H7N2O2S |
243 |
262-264 |
70 |
59.25(58.98) |
2.88(2.67) |
11.52(11.24) |
13.16(12.94) |
Table 2 Analytical & physicochemical data of the synthesized compounds (A1-A12)
QSAR
Intercorrelation between the captions in the final equations is less than 0.2. Best equations correlating Log (% Immobility) with descriptors for series (A1-A12) generated are conferred in Table 3.
|
Compound code |
Immobility time(±SD) |
% Immobility (±SD) |
||||
|
|
1 Hr |
5 Hr |
24 Hr |
1 Hr |
5 Hr |
24 Hr |
|
A1 |
160.5±0.23 |
159.5±0.31 |
169.5±0.12 |
93.02±0.16 |
87.39±0.09 |
86.92±0.38 |
|
A2 |
150.5±0.16 |
153±0.18 |
160.5±0.08 |
87.50±0.12 |
83.83±0.23 |
82.30±0.09 |
|
A3 |
165±0.12 |
169.5±0.19 |
174±0.27 |
95.93±0.10 |
92.87±0.18 |
89.23±0.29 |
|
A4 |
142.5±0.34 |
155±0.27 |
163.5±0.41 |
82.84±0.09 |
84.93±0.06 |
83.84±0.10 |
|
A5 |
140.5±0.11 |
155.5±0.08 |
161.5±0.19 |
81.68±0.21 |
85.20±0.27 |
82.82±0.29 |
|
A6 |
159±0.27 |
159.5±0.3 |
168±0.62 |
92.44±0.11 |
87.39±0.17 |
86.15±0.18 |
|
A7 |
148.5±0.26 |
160.5±0.28 |
169.5±0.09 |
86.33±0.27 |
87.94±0.29 |
86.92±0.30 |
|
A8 |
157.5±0.17 |
162.5±0.19 |
172±0.21 |
91.56±0.29 |
89.04±0.06 |
88.20±0.13 |
|
A9 |
149.5±0.33 |
153.5±0.28 |
163.5±0.45 |
86.91±0.39 |
84.10±0.29 |
83.84±0.28 |
|
A10 |
163.5±0.18 |
169.5±0.14 |
175.5±0.46 |
95.05±0.26 |
92.87±0.23 |
90.0±0.38 |
|
A11 |
161.5±0.06 |
164.5±0.50 |
172±0.08 |
93.89±0.1 |
90.13±0.2 |
88.20±0.16 |
|
A12 |
161±0.21 |
162.5±0.32 |
169.5±0.39 |
93.60±0.24 |
89.04±0.25 |
86.92±0.3 |
|
Control |
172±0.28 |
182.5±0.29 |
195±0.4 |
100±0.11 |
100±0.26 |
100±0.36 |
|
Imipramine (std.) |
136.5±0.07 |
150.5±0.10 |
154.5±0.06 |
79.36±0.09 |
82.49±0.67 |
79.26±0.24 |
Table 3 Antidepressant activities of the compounds
Significance of the terms –
N= No. of Molecules,
s = standard error --- less is better,
r = correlation coefficient – higher is better > 0.7,
r2cv = cross validated r2 - higher is better > 0.5,
F Value = higher is better
Observed and predicted data and graphs are conferred in Table 4 & 5 (Figure 4a & 4b) and Graph I for Series
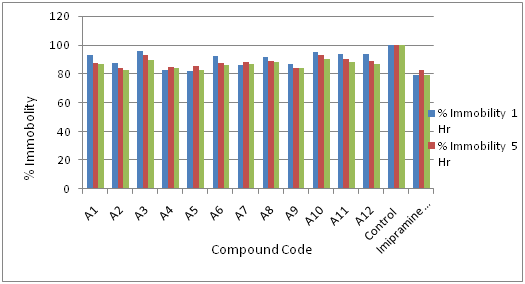
Figure 3 Antidepressant-like effects of Coumarin derivatives test compounds in FST Data are presented as compared to control group.(Compounds code vs % Immobility).
|
Sr. No. |
Equation |
N |
S |
r |
r2 |
r2cv |
F |
|
Series (A1-A12) |
Y = -0.199*X3 - 0.229*X1 - 1.553*X2-12.575 |
12 |
0.06438 |
0.9835 |
0.9674 |
0.9503 |
8.14 |
Table 4a Equations generated between Log (% Immobility) and descriptors
Where
Y = Log (% Immobility)
X1 = ClogP-
X2 = VAMP HOMO (Whole Molecule)
X3 = Dipole Moment Z Component (Whole Molecule)
X4 = Inertia Moment 2 Length (Whole Molecule)
|
Compound No. |
Observed Value |
Predicted Value |
Residual Value |
|
A1 |
1.96857 |
1.96658 |
0.00199 |
|
A2 |
1.942 |
1.94568 |
-0.00368 |
|
A3 |
1.98195 |
1.97986 |
0.00209 |
|
A4 |
1.91824 |
1.91123 |
0.00701 |
|
A5 |
1.91211 |
1.90321 |
0.0089 |
|
A6 |
1.96585 |
1.97684 |
-0.01099 |
|
A7 |
1.936116 |
1.93424 |
0.001876 |
|
A8 |
1.9617 |
1.95987 |
0.00183 |
|
A9 |
1.93906 |
1.94102 |
-0.00196 |
|
A10 |
1.97795 |
1.97988 |
-0.00193 |
|
A11 |
1.97261 |
1.96892 |
0.00369 |
|
A12 |
1.97127 |
1.96892 |
0.00235 |
Table 4b Observed and predicted log (% Immobility) value data for 12 compounds
The range of demographic interpretation of the equations is accepted. Less deviation with the correlation coefficient is high. The residual value for each sequence also is less indicating good guessing power of models. From equation it is observed that two electronic specification Dipole Moment Z Component (Whole Molecule) and VAMP HOMO (Whole Molecule) as well as one static parameter Inertia Moment 2 Length (Whole Molecule) contribute (-0.227, -1.469 and -0.414 respectively) negatively for the action so electron eliminate and less heavy groups may upgrade the activity (%1 Immobility). Synthetic method and scientific applications of coumarins for the treatment of several defects were seriously discussed.
In the present research, attempt has been made to present synthetic strategies, reactions, in the structural skeleton of the compounds for their antidepressant activity. At dose (25mg/kg) the compounds shows good antidepressant activity. The compounds codes (A4, A5 and A9) showed excellent activity. Lastly, the boost result of the antidepressant activity showed by these compounds may be of significant for further structural alteration to the chief compound and next level studies in the desire of finding a new great antidepressant instruction.
None.
Authors declare that there is no conflict of interest.

©2016 Dighe, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.