Journal of
eISSN: 2473-0831


Research Article Volume 2 Issue 3
Correspondence: Lakshmi Sivasubramanian, Department of Pharmaceutical Analysis, Sri Venkateswara College of Pharmacy, JNTUA University, RVS Nagar, Chittoor - 517127, AP, India
Received: April 23, 2016 | Published: May 9, 2016
Citation: Sivasubramanian L, Lakshmi KS (2016) Spectrophotometric Multicomponent Analysis of Irbesartan, Hydrochlorothiazide and Ramipril in Pharmaceutical Formulations by Chemometric Techniques. J Anal Pharm Res 2(3): 00019. DOI: 10.15406/japlr.2016.02.00019
Partial Least Squares (PLS) and Principal Component Regression (PCR) methods were proposed for the spectrophotometric analysis of a ternary mixture consisting of Irbesartan, Hydrochlorothiazide and Ramipril without prior separation. In these chemometric techniques the measurements of absorbance values were realized in the spectral range from 200-350 nm in the intervals of Dl=1nm at the 151 wavelengths in the zero order, first and second order spectra of the different ternary mixtures of these ingredients in 0.1 M NaOH. The prepared calibrations of both techniques using the absorbance data and concentration matrix data sets were used to predict the concentrations of the unknown concentrations of Irbesartan, Hydrochlorothiazide and Ramipril in their ternary mixture. The techniques were successfully applied to pharmaceutical formulations marked in India.
Keywords: irbesartan, hydrochlorothiazide, ramipril, chemometrics, simultaneous estimation, calibrations, absorbance, chemometric, spectrophotometric
PLS, partial least squares; PCR, principal component regression; IRB, irbesartan; HCZ, hydrochlorothiazide; RAM, ramipril; HPLC, high performance liquid chromatography; PLS, partial least squares regression; SEP, standard error prediction
For the determination of Irbesartan (IRB), Hydrochlorothiazide (HCZ) and Ramipril (RAM), different analytical methods such as spectrophotometry,1-6 spectrofluorimetry,7,8 chemometrics,9-14 high Performance Liquid Chromatography (HPLC),15-35 LC-MS,36-41 capillary zone electrophoresis42 and High performance thin layer chromatography (HPTLC)43-47 were reported individually or in combination with other drugs. But no method was reported so far for the simultaneous analysis of these drugs in ternary mixture. In comparison with conventional univariate methods, Multivariate spectrophotometric methods provide several advantages as it includes multiple variables for data analysis. These methods overcome the limitations of conventional techniques thereby applied to the simultaneous quantification of multiple analytes in mixtures.
For quantitative analysis of many pharmaceutical formulations, multivariate calibration methods like Partial Least Squares Regression (PLS) and Principal Component Regression (PCR) are predominantly used. These methods are based on the association between matricial algorithms and calibration data and the theoretical base of these methods are not fully explored by authors.
Due to the spectral overlapping simultaneous estimation of three components becomes difficult by conventional methods. Derivative spectrophotometry offers an effective quantification of multicomponent samples from their mixtures, as they resolve the common overlapping seen in the fundamental spectra. Hence derivative spectrophotometry offers greater sensitivity than the normal spectrophotometry in the simultaneous determination of two components without prior separation. An attempt has been made to overcome such spectral overlapping felt by three components in a ternary mixture by the combined use of derivative spectrophotometry and chemometric techniques.
Two multivariate procedures such as PCR and PLS algorithms are reported for the ternary mixture of drugs of interest. An effort was also made to compare the regression methods for fundamental spectra with that of first and second derivative spectra. From a calibration set comprising of 25 reference mixtures of selected analytes, the calibration models were built using a novel experimental design. In order to verify the prediction ability of the defined models, and external validation test was performed by using prediction set comprising of 10 synthetic mixtures of three analytes. The proposed methods were finally applied to the quantitative analysis of commercial samples containing one to three drugs to confirm their effectiveness in the routine quality control analysis of the real samples.
Apparatus
A Perkin Elmer (Lamda 25) spectrophotometer controlled by UV winlab software and equipped with 1 cm pathlength quartz cell was used for the spectral data acquisition. The chemometric procedure was carried out using MATLAB software version 7.5 (The Mathworks) and PLS toolbox version 5.0 (Eigen Vector Technologies).
Chemicals
IRB, HCZ and RAM reference standards were kindly supplied by Madras Pharmaceuticals, Chennai, India. The tablets IROVEL H, Sun Pharmaceuticals, XARB-H, Hetero Labs (both containing 150 mg of IRB and 12.5 mg of HCZ) and Cardace#2.5, Aventi Pharma (containing 2.5 mg of RAM and 12.5 mg of HCZ) were procured from local pharmacies. All other chemicals were of analytical reagent grade and procured from SD Fine chemicals, Mumbai, India.
Standard solutions
Standard stock solutions (1000μg/ml) of IRB, HCZ and RAM were prepared separately in the diluent 0.1M NaOH and water in the ratio 20:80. These solutions were taken and then diluted to 10 ml with water to give a final analyte concentration desired.
Sample preparation
Ten tablets were weighed and finely powdered in a mortar. A quantity of the powder equivalent to one tablet was accurately weighed and transferred into a 100ml volumetric flask including the diluent. The flask was sonicated for 15mins and diluted to the mark with diluents. An aliquot of the solution was centrifuged at 5000 rpm for 10mins. Appropriate amount of clear supernatant was transferred into a 10 ml flask and diluted with water. Then the absorbance values were measured.
Multivariate calibration methods
In multivariate calibration methods, a calibration model is built using the absorbance values and a concentration data set. These methods are designed based on the relationship between matrices of chemical data. Initially a calibration model is built using a chemical data set i.e. absorbance values and a concentration data set. The constructed calibration model is then used to estimate the concentration of the unknown samples in the prediction set. In the PLS and PCR methods, the original variables are transformed in to smaller number of orthogonal variables called factors or principal components which are in turn linear with the original variables.
From the prepared reference samples, a relationship between spectral and concentration data, representing the variables of the system can be built using the multivariate calibration techniques. Based on the regression method, the PCs and scores can be built specific to the new matrix. The PCs corresponds to the absorptivity values of the samples at various concentration and wavelength values, whereas the scores corresponds the numerical coefficients. This allows to build the mathematical model representing the reference spectra and able to predict the component concentrations of new samples.
Determination of PC number
The prediction power of the selected methods can be improved by determining the optimal number of factors. This can be achieved by employing a full cross validation also known as leave-one-out method. This method employs leaving one sample at a time from the calibration step and performs the calibration with all other samples. The concentration of the sample removed is then predicted with the obtained model. This step is in turn repeated for each sample considered. The procedure can be repeated after fixing a different number of factors. The standard error prediction (SEP) was chosen as an optimizing criterion to select the optimal number of factors. SEP represents an estimate of the error involved in the assay of external samples by using the model. Its value depends on the number of factors used for that calibration. The number of factors giving the minimal SEP was selected as the optimal number of factors.
Where denotes the added drug concentration, Ci is the predicted drug concentration and n represents the total number of synthetic mixtures.
Data Processing and model building
The UV absorption spectra of standard IRB, HCZ and RAM in 0.1 M NaOH is recorded in the range of 200-350nm in ordinary mode (Figure 1a), First derivative mode (Figure 1b) and second derivative mode (Figure 1c). Specific wavelengths for these components could not be signaled out from all the recorded spectra. Hence multivariate calibration methods appeared to be ideal to overcome the drawbacks, as the entire information from the full spectra is being utilized by these techniques.
A concentration of 25 mixtures of three compounds in the range of 0.5-3µg/ml of IRB and HCZ and 1-6 µg/ml of RAM were prepared in 0.1M NaOH (Table 1). The fundamental, first derivative and second derivative spectra of the proposed concentration set were recorded in the 200 -350 nm wavelength range. The absorption values of spectra of the concentration set were measured at the 151 wavelength points with Dl = 1 nm in the spectral region of 200 -350 nm. The concentration set and absorption data were considered as Y-block (25×3) and X-block (25×151) for the construction of PLS calibration using cross-validation procedure to reach the best calibration model. The calculations were done with PLS Toolbox 5.0 and the optimal factors were selected.
|
Calibration set (µg/ml) |
Prediction set (µg/ml) |
||||
|
IRB |
HCZ |
RAM |
IRB |
HCZ |
RAM |
|
0.5 |
0.5 |
5 |
2 |
0.5 |
1.5 |
|
1 |
0.5 |
4 |
2 |
1 |
2.5 |
|
1.5 |
0.5 |
3 |
1 |
1.5 |
2 |
|
2 |
0.5 |
2 |
1.5 |
2 |
2.5 |
|
0.5 |
1 |
5 |
0 |
2.5 |
1.5 |
|
1 |
1 |
4 |
0.5 |
3 |
4 |
|
1.5 |
1 |
3 |
1.5 |
3 |
4 |
|
2 |
1 |
2 |
0 |
0 |
3 |
|
0.5 |
0.5 |
5 |
0 |
1 |
6 |
|
1 |
1.5 |
4 |
1 |
2 |
1.5 |
|
1.5 |
1.5 |
3 |
- |
- |
- |
|
2 |
1.5 |
2 |
- |
- |
- |
|
0 |
2 |
0 |
- |
- |
- |
|
0.5 |
2 |
5 |
- |
- |
- |
|
1 |
2 |
4 |
- |
- |
- |
|
1.5 |
2 |
3 |
- |
- |
- |
|
0.5 |
2.5 |
5 |
- |
- |
- |
|
1 |
2.5 |
4 |
- |
- |
- |
|
1.5 |
2.5 |
3 |
- |
- |
- |
|
2 |
2.5 |
2 |
- |
- |
- |
|
0.5 |
3 |
1 |
- |
- |
- |
|
1 |
3 |
1.5 |
- |
- |
- |
|
0 |
0 |
6 |
- |
- |
- |
|
3 |
0 |
0 |
- |
- |
- |
|
1.5 |
3 |
2 |
- |
- |
- |
Table 1 Concentrations of IRB, HCZ and RAM used as Calibration and Prediction sets
The PCR and PLS models obtained by using this training set were validated by full cross-validation and the SEP values were calculated each time that a new factor was added to the models. Table 2 & Figure 2 shows the selected numbers of factors and the corresponding SEP values. The square of the correlation coefficient (R2), which indicates the fraction of the total variance explained by the models, is also reported. The prediction values for IRB, HCZ and RAM in both models were found satisfactory (Figure 3).
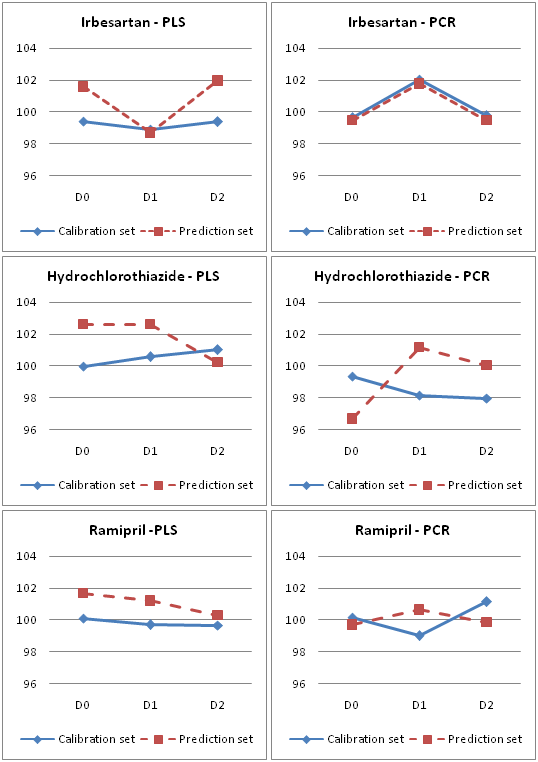
Figure 2 Comparison of Mean Recovery of PLS and PCR models for Fundamental (D0), First Derivative (D1) and Second Derivative (D2) Spectra.
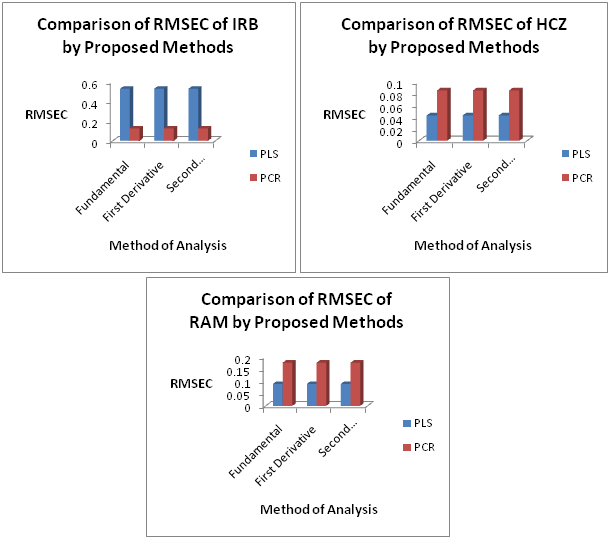
Figure 3 Comparison of Root Mean Square Error of Calibration (RMSEC) of PLS and PCR models for Fundamental (D0), First Derivative (D1) and Second Derivative (D2) Spectra.
|
Drug |
Parameter |
PLS |
PCR |
||||
|
D0 |
D1 |
D2 |
D0 |
D1 |
D2 |
||
|
IRB |
Mean |
99.436 |
98.919 |
99.396 |
99.634 |
99.634 |
99.634 |
|
RMSEC |
0.5298 |
0.5298 |
0.5298 |
0.1262 |
0.1262 |
0.1262 |
|
|
R2 |
0.9943 |
0.9943 |
0.9943 |
0.9685 |
0.9685 |
0.9685 |
|
|
HCZ |
Mean |
99.979 |
100.576 |
101.018 |
99.34 |
99.34 |
99.34 |
|
RMSEC |
0.0431 |
0.0431 |
0.0431 |
0.0857 |
0.0857 |
0.0857 |
|
|
R2 |
0.9979 |
0.9979 |
0.9979 |
0.9919 |
0.9919 |
0.9919 |
|
|
RAM |
Mean |
100.111 |
99.695 |
99.689 |
100.165 |
100.165 |
100.165 |
|
RMSEC |
0.0892 |
0.0892 |
0.0892 |
0.1761 |
0.1761 |
0.1761 |
|
|
R2 |
0.9968 |
0.9968 |
0.9968 |
0.9877 |
0.9877 |
0.9877 |
|
Table 2 Statistical Parameters calculated from application of PLS and PCR methods to calibration samples
Mean, Mean of 25 samples of calibration set; RMSEC, root mean square error of calibration set;
R2, correlation coefficient obtained by plotting amount present against amount estimated.
Application of PLS and PCR models to the Prediction set
In order to perform an external validation for the proposed PLS and PCR models, a set of 10 synthetic samples containing ternary and binary component mixtures of these selected drugs were prepared. The mixtures were prepared in the same concentration range as in the calibration set (Table 1). Such constructed prediction set was used to test the prediction ability of the models. The application of proposed PLS and PCR models to the prediction set gave the following results and are indicated in Table 3 & Figure 4.
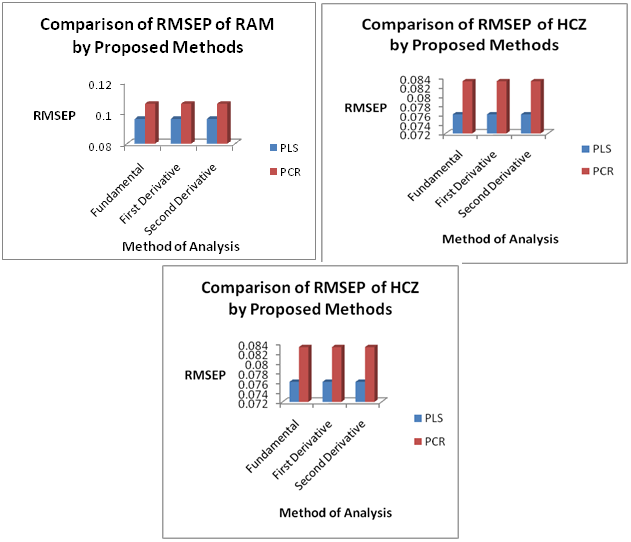
Figure 4 Comparison of Root Mean Square Error of Prediction (RMSEP) of PLS and PCR models for Fundamental (D0), First Derivative (D1) and Second Derivative (D2) Spectra.
|
Drug |
Parameter |
PLS |
PCR |
||||
|
D0 |
D1 |
D2 |
D0 |
D1 |
D2 |
||
|
IRB |
Mean % Recovery |
101.5939 |
101.5939 |
101.5939 |
99.0195 |
99.0195 |
99.0195 |
|
RMSEP |
0.1481 |
0.1481 |
0.1481 |
0.0808 |
0.0808 |
0.0808 |
|
|
SD |
5.8949 |
5.8949 |
5.8949 |
4.4397 |
4.4397 |
4.4397 |
|
|
HCZ |
Mean % Recovery |
102.6194 |
102.6194 |
102.6194 |
96.6958 |
96.6958 |
96.6958 |
|
RMSEP |
0.0761 |
0.0761 |
0.0761 |
0.0832 |
0.0832 |
0.0832 |
|
|
SD |
4.8994 |
4.8994 |
4.8994 |
6.3584 |
6.3584 |
6.3584 |
|
|
RAM |
Mean % Recovery |
101.6947 |
101.6947 |
101.6947 |
99.6934 |
99.6934 |
99.6934 |
|
RMSEP |
0.0961 |
0.0961 |
0.0961 |
0.1059 |
0.1059 |
0.1059 |
|
|
SD |
2.647 |
2.647 |
2.647 |
3.7579 |
3.7579 |
3.7579 |
|
Table 3 Accuracy (% recovery) and Precision (SD) results from application of optimized PLS and PCR models on the prediction set
Mean % Recovery, mean of 10 samples of prediction set; RMSEP, root mean square error of prediction set; SD, standard deviation
The results of PLS model includes mean % recovery ranging from 98.919 to 101.018 for all the three drugs in the calibration set and from 98.704 to 102.619 in the prediction set. The SD values were found to increase with increasing spectral mode in the calibration set but seem to decrease with increasing spectral mode in the prediction set. R2 value ranges from 0.9932-0.9979 in the calibration set and from 0.9635-0.9988 in the prediction set. RMSEC value ranges from 0.0431-0.1302 for all three drugs in the calibration set. RMSEP value ranges from 0.0469-0.1481 for all three drugs in the prediction set.
The results of PCR model includes mean % recovery ranging from 98.919 to 101.018 for all the three drugs in the calibration set and from 98.704 to 102.619 in the prediction set. The SD values were found to increase with increasing spectral mode in the calibration set but seem to decrease with increasing spectral mode in the prediction set. R2 value ranges from 0.9932-0.9979 in the calibration set and from 0.9635-0.9988 in the prediction set. RMSEC value ranges from 0.0431-0.1302 for all three drugs in the calibration set. RMSEP value ranges from 0.0469-0.1481 for all three drugs in the prediction set.
Analysis of commercial formulations
The proposed PLS and PCR models were applied to the assay of binary pharmaceutical formulations. The assay results are summarized in Table 4 & Figure 5. A good coincidence was observed between experimental results and label claim of the pharmaceutical formulations. Application of models from fundamental to second derivative spectra gave recovery between 98.72 to 101.20% and SD of not more than 1.0. No interference from the excipients was observed.
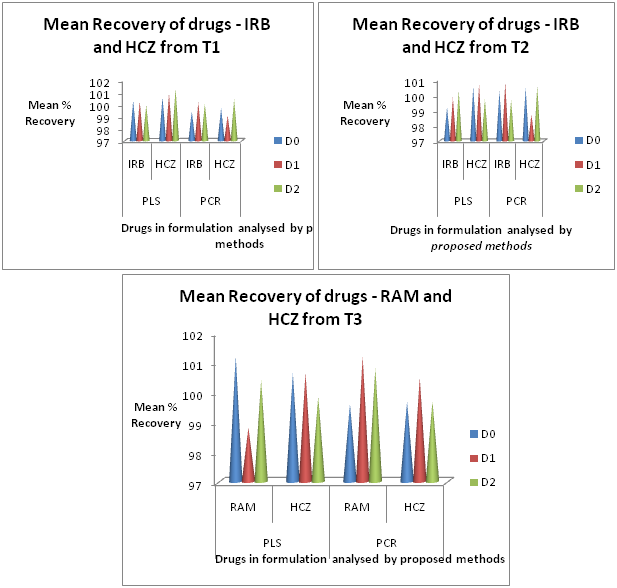
Figure 5 Comparison of % Recovery of drugs from formulations by PLS and PCR models for Fundamental (D0), First Derivative (D1) and Second Derivative (D2) Spectra.
|
Method |
Tablet |
Mode |
IRB |
HCZ |
||
|
Amount Found* (mg/tab) ± SD |
% Recovery |
Amount Found* (mg/tab) ± SD |
% Recovery |
|||
|
PLS |
T1 |
D0 |
150.45 ± 0.21 |
100.3 |
12.57 ± 0.11 |
100.56 |
|
D1 |
150.32 ± 0.11 |
100.21 |
12.61 ± 0.13 |
100.88 |
||
|
D2 |
149.88 ± 0.08 |
99.92 |
12.66 ± 0.13 |
101.28 |
||
|
T2 |
D0 |
148.82 ± 0.05 |
99.21 |
12.57 ± 0.05 |
100.56 |
|
|
D1 |
149.76 ± 0.12 |
99.84 |
12.59 ± 0.11 |
100.72 |
||
|
D2 |
150.43 ± 0.04 |
100.28 |
12.47 ± 0.03 |
99.76 |
||
|
PCR |
T1 |
D0 |
149.11 ± 0.11 |
99.4 |
12.46 ± 0.04 |
99.68 |
|
D1 |
150.21 ± 0.23 |
100.14 |
12.38 ± 0.13 |
99.04 |
||
|
D2 |
150.08 ± 0.06 |
100.05 |
12.55 ± 0.12 |
100.4 |
||
|
T2 |
D0 |
150.42 ± 0.03 |
100.28 |
12.56 ± 0.06 |
100.48 |
|
|
D1 |
151.22 ± 0.07 |
100.81 |
12.34 ± 0.23 |
98.72 |
||
|
D2 |
149.53 ± 0.03 |
99.68 |
12.57 ± 0.04 |
100.56 |
||
|
Method |
Tablet |
Mode |
RAM |
HCZ |
||
|
Amount Found* (mg/tab) ± SD |
% recovery |
Amount Found* (mg/tab) ± SD |
% Recovery |
|||
|
PLS |
T3 |
D0 |
2.53 ± 0.04 |
101.2 |
12.58 ± 0.11 |
100.64 |
|
D1 |
2.47 ± 0.03 |
98.8 |
12.58 ± 0.03 |
100.64 |
||
|
D2 |
2.51 ± 0.03 |
100.4 |
12.48 ± 0.04 |
99.84 |
||
|
PCR |
T3 |
D0 |
2.49 ± 0.04 |
99.6 |
12.46 ± 0.03 |
99.68 |
|
D1 |
2.53 ± 0.03 |
101.2 |
12.56 ± 0.02 |
100.48 |
||
|
D2 |
2.52 ± 0.02 |
100.8 |
12.46 ± 0.04 |
99.68 |
||
Table 4 Assay Results of optimized PLS and PCR models on Pharmaceutical preparations
*Average of six estimations
T1 - IROVEL H - Sun Pharmaceuticals, J&K, Irbesartan-150 mg and Hydrochlorothiazide-12.5 mg
T2 - XARB H - Hetero Labs, HP, Irbesartan-150 mg and Hydrochlorothiazide-12.5 mg
T3 - Cardace # 2.5 - Aventis Pharma, Goa, Ramipril-2.5 mg and Hydrochlorothiazide-12.5 mg
Table Abbreviations:
PLS, partial least square; PCR, principal component regression; IRB, irbesartan; HCZ, hydrochlorothiazide; RAM, ramipril; D0, fundamental/zero order; D1, first order; D2, second order; RMSEC, root mean square error of calibration; RMSEP, root mean square error of prediction; SD, standard deviation; R2, correlation coefficient
The spectrophotometric data of complex mixtures results in severe spectral overlapping thereby imposing difficulty in simultaneous analyses. Multivariate methods such as PCR and PLS overcomes the difficulty and proves to be valid analytical tools for quantitative estimation of analytes from complex mixtures. The prediction ability of the multivariate methods can be further magnified by acquisition of derivative spectra. The application of PLS and PCR methods on zero to second order derivative spectra of ternary mixtures of IRB, HCZ and RAM has been accomplished. Very satisfactory results were obtained when the optimized models were applied to the synthetic mixtures and commercial formulations. According to these studies, multivariate calibration methods (PLS and PCR) coupled with derivative spectral data can be recommended as a very suitable choice to resolve severe overlapped absorption spectra of drug mixtures. This approach is simple in application, inexpensive, requires an easy treatment of the samples and provides reliable analytical results.
The authors are thankful to the management of SRM University for providing the facilities to carry out the work. The authors are also thankful to Madras Pharmaceuticals, Chennai for providing the standard drugs as gift samples.
Authors declare that there is no conflict of interest.

©2016 Sivasubramanian, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.