Journal of
eISSN: 2373-6437


Research Article Volume 13 Issue 1
Lourdes Hospital, India
Correspondence: Shoba Philip, Head of Department of Anaesthesiology & Critical Care, Lourdes Hospital, Postgraduate Institute of Medical Science & Research, Pachalam, Cochin, Kerala, India, Tel 9847920355
Received: December 22, 2020 | Published: February 10, 2021
Citation: Augustine A, Philip S. Effect of perioperative intravenous lignocaine infusion on postoperative pain in laparoscopic cholecystectomy. J Anesth Crit Care Open Access. 2021;13(1):22‒28. DOI: 10.15406/jaccoa.2021.13.00465
Background and Aims: Postoperative pain delays recovery after laparoscopic cholecystectomy which is done as a daycare surgery. Opioid analgesia carries adverse effects like nausea, vomiting and respiratory depression which can affect postoperative recovery. Our aim was to compare lignocaine infusion with saline infusion as perioperative ERAS measure for postoperative analgesia in laparoscopic cholecystectomy.
Method: After obtaining ethical clearance, 40 patients undergoing laparoscopic cholecystectomy were randomised by computer generated codes in to two groups A and B. In group B two subjects were moved out of study, so two in group A were also excluded to make the two groups equal. Group A received saline and group B received 2mg/kg/h lignocaine infusion intravenously which were stopped at the end of the surgery. Postoperative pain was assessed by NRS score at definite time intervals 0 (admission to postoperative anesthesia care unit), 2nd, 4th, 6th, 8th, 10th, 12th and 24th hours. Time at which first rescue analgesia was requested, total analgesic requirements, mean NRS score and side effects were noted.
Results: At every time interval, patients in lignocaine group had delayed time to request for rescue analgesia, less analgesic requirements and mean NRS. (P value <0.05). No side effects or signs of toxicity were noted in lignocaine group.
Conclusion: Perioperative lignocaine infusion is an efficient, safe and cost-effective armamentarium in implementing ERAS in laparoscopic cholecystectomy.
Keywords: Lignocaine, ERAS, Postoperative analgesia, Laparoscopic cholecystectomy
Postoperative pain is a significant factor delaying recovery and discharge after laparoscopic cholecystectomy which is usually performed as a day care surgery.1 This pain can cause atelectasis, sympathetic over activity, prolonged hospital stays and decreased patient satisfaction.2 Moreover inadequate treatment of acute postoperative pain can predispose to development of chronic pain.3 Opioids used for postoperative pain relief have adverse effects like nausea, vomiting, respiratory depression which can significantly affect recovery and discharge.4 Multimodal analgesia has been tried as a part of Enhanced Recovery after surgery (ERAS) in laparoscopic cholecystectomy.5
Perioperative intravenous infusion of preservative free lignocaine has been proposed to reduce postoperative pain significantly by inhibiting proinflammatory mediators. Lignocaine inhibits priming of polymorphonuclear neutrophils preventing its activation and subsequent inflammation.6 Our randomised double blinded placebo-controlled study aimed at assessing the efficacy of perioperative infusion of preservative free lignocaine on postoperative pain in laparoscopic cholecystectomy and to look for any adverse effects associated with lignocaine administration.
After institutional ethical and scientific committee clearance (LH/EC/2018-10) and explaining the procedure, with written informed consent, 40 patients in the age group of 18-65 years of either gender, with a weight between 50 to 80 kg, with American society of anaesthesiologists physical status I and II, who were scheduled to undergo elective laparoscopic cholecystectomy under general anaesthesia with endotracheal intubation with a duration between 90 to 180 min were recruited in this prospective, double-blind, randomised comparative study. Exclusion criteria included patients with significant hepatic, renal, cardiac and respiratory diseases (ASAIII and more), patients allergic to lignocaine, patients who have taken nonsteroidal anti-inflammatory drugs within 24 hours of surgery, patients with history of drug and alcohol abuse and patients in whom laparoscopy was converted to open technique.
A sample size of 16 patients per group was required to detect a significant change in NRS score at 6th postoperative hour (based on previous study by Xiaoli song et al.)7 after drug administration and with a power of 95% at 1% significance level. The total sample size was obtained as 32 but to account for any fallout we decided to allot 20 patients per group. (total 40). Patients were randomly allocated in to two groups with the help of computer-generated randomisation techniques and allocation details and method of preparation of the solution was kept in an opaque sealed envelope.
After a thorough preoperative assessment the patients were educated as to how to use the Numerical Rating Scale and to mark the score accordingly, 0-no pain to 10-worst pain. After fasting for solids for 8 hours, 100ml clear fluid was allowed 2 hours preoperatively. All patients were premedicated with Tab. Ranitidine 150mg and Tab. Ondansetron 4 mg on previous night and on the morning of surgery and were given a test dose of lignocaine 0.1ml intradermally and were assessed for induration or any signs or symptoms of lignocaine allergy. Intravenous line was started using 18G cannula and intravenous normal saline was started at the rate of 4ml/kg/hr in both groups. As per sealed envelopes anaesthesiologist in the operation theatre, not involved in the study prepared the infusion as per groups allocated- control group with saline and study group with lignocaine. Preoperatively standard monitors like pulse oximeter, electrocardiogram and non-invasive blood pressure and temperature were monitored and baseline values were noted. All patients were preoxygenated with 100% oxygen for 3 minutes and in both groups 0.02mg/kg intravenous midazolam, 0.02 mg/kg intravenous glycopyrrolate, 2mcg/kg intravenous fentanyl followed by1.5 mg/kg intravenous lignocaine and induction with 2mg/kg intravenous propofol was done as per our hospital protocol. Intravenous lignocaine bolus was given to time intubation 90 seconds later to tackle hemodynamic responses to laryngoscopy and intubation as per hospital protocol. After loss of consciousness, ventilation was manually assisted. 2mg/kg intravenous succinylcholine was given to facilitate elective endotracheal intubation with proper sized endotracheal tube. After confirmation of tube placement by 5point auscultation and end tidal capnometry, anaesthesia was maintained by 50% Oxygen 50% Nitrous Oxide and sevoflurane 1-1.5% adjusted to achieve a MAC of 0.8, along with 0.5 mg/kg intravenous atracurium at 6L/min for 6 minutes. Low flow technique was adopted was adopted (total flow 1L/min). Immediately after intravenous atracurium, 2mg/kg/hr lignocaine made up to a volume of 10ml using 0.9% normal saline was given using an infusion pump FRESENIUS ™ in group B (study group) whereas in group A (control group) 10 ml normal saline was given using an infusion pump for blinding at 2ml/kg/h. In all patients, nasogastric tube was put. Patient’s hemodynamic and respiratory parameters like heart rate, non-invasive blood pressure, respiratory rate, end tidal CO2 and patient's temperature were monitored throughout the procedure.
Mechanical ventilation was adjusted to achieve end tidal CO2 at 35-45 mm Hg. Analgesia was supplemented with intravenous paracetamol 1gm infusion in both the groups. Injection ondansetron (0.08mg/kg) intravenous and dexamethasone 0.2mg/kg intravenous was given in both the groups towards the end of surgery. None of the patients were given any other form of local anaesthetic infiltration. The infusion of saline or lignocaine was continued till the end of surgery. sevoflurane and nitrous oxide were discontinued and oxygen flow was increased to 6L/min at the end of surgery, nasogastric tube was removed after suction and neuromuscular blockade was reversed with 0.05mg/kg intravenous neostigmine and 0.08mg/kg intravenous glycopyrrolate and trachea was extubated. The patient was then transferred to post anaesthesia care unit (PACU). The time of arrival in PACU was taken as zero hour. Patient was administered O2 via face mask and was monitored with pulse oximeter, non-invasive blood pressure monitoring and electrocardiogram. Once patient achieved a Modified Aldrete’s score of 9 (recovery score) patients were shifted out from PACU to concerned ward.
Outcome measures
The primary investigator assessed the patients in both groups at definite time intervals starting from zero hour.
Pulse rate and mean arterial pressure were monitored at 0, 2, 4, 6, 8, 10, 12 and 24 hours postoperatively. Patients were also observed for side effects like nausea, vomiting, and respiratory depression and signs of toxicity like perioral numbness, tinnitus, light headedness, seizures, hypertension, hypotension, cardiac arrhythmias by the primary investigator.
All data collected were recorded on the data collection form prepared for the study and the results thus obtained were subjected to statistical analysis. SPSS version 20 was used for statistical analysis. Chi-square test and Fisher's exact test were performed to compare qualitative (categorical) variables between the two groups. Independent sample t-test (Student's t-test) was performed to compare quantitative (continuous) variables between the two groups. p-value less than 0.05 was taken as statistically significant.
Forty patients were allocated to the study, two patients in the control group were converted to open hence two were excluded from lignocaine group to make the two groups equal (Figure 1).
Patients in the two groups were comparable in age, gender, weight, height, ASA physical status and duration of anaesthesia (Table 1).
|
Characteristics |
Group A CONTROL |
Group B LIGNOCAINE |
p value |
||
|
Age (years)
|
46.17(12.133) |
42.10(11.346) |
0.293 |
||
|
Gender (male/female) |
4/14 |
5/13 |
0.841 |
||
|
Weight (in kg) |
68.017(11.124) |
67.730(9.737) |
0.933 |
||
|
Height (in cm) |
161.5(11.5160) |
162.575(10.8837) |
0.769 |
||
|
ASA PS grade (I/II) |
I-10 |
II-8 |
I-15 |
II-3 |
0.070 |
|
Anaesthesia duration |
88.0(6.686) |
87.17(8.706) |
0.749 |
||
Table 1 Comparison of patient characteristics between the two groups
Mean NRS score over 24 h was significantly less in lignocaine group compared to control group (p =0.001) (Table 2, Figure 2).
|
Group |
n |
Mean NRS score |
Std. deviation |
p value |
|
Control |
18 |
3.006 |
0.3469 |
0.001 |
|
Lignocaine |
18 |
1.61 |
0.394 |
Table 2 Table showing mean NRS score over 24hour in Lignocaine and control groups
Analysis of postoperative NRS score at 0hour, 2hour, 4hour, 6hour, 8hour, 10hour, 12 hour and 24hour postoperatively showed significant reduction in pain for lignocaine group compared to the control group.
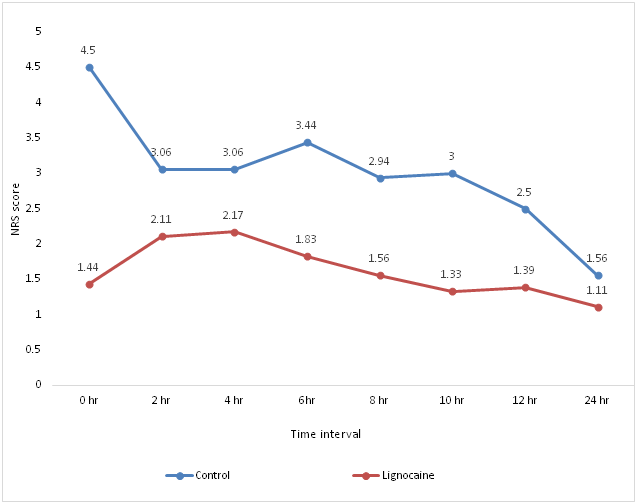
Figure 2 Graphical analysis of postoperative NRS score at definite time intervals between the two groups.
Comparison of number of patients requiring rescue analgesia showed that 18 subjects in the control group required first rescue analgesia whereas only 6 in lignocaine group required rescue analgesia, 18 patients in control group required second rescue analgesic and 8 subjects in the control group required third rescue analgesic; whereas none of the subjects in lignocaine group required second or third rescue analgesic. Fischer's exact test was done and gave a p value of 0.03. This shows that lignocaine reduced analgesic requirements in a statistically significant number of patients (Table 3, Figure 3).
|
Group |
Rescue 1 |
Rescue 2 |
Rescue 3 |
p value |
|
Control |
18 |
18 |
8 |
0.033 |
|
Lignocaine |
6 |
0 |
0 |
Table 3 Comparison of number of patients receiving rescue analgesia in the two groups
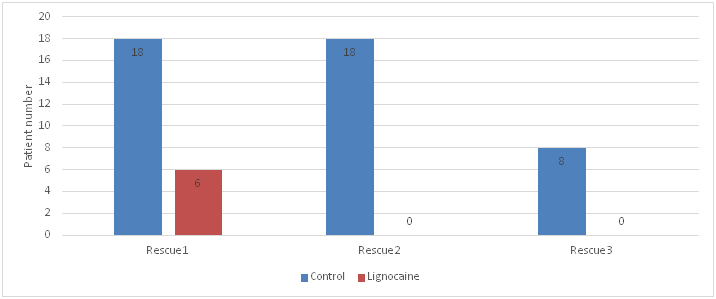
Figure 3 Graphical representation of number of patients receiving rescue analgesia in the two groups.
Comparison of time for rescue analgesia showed that time for first rescue analgesia in lignocaine group was 270 minutes compared to 10 minutes in control group, with significant p value 0.00. 18 patients in control group required second rescue agent at 287.58 minutes and 8 in control group required third rescue agent at 581.25 minutes. This showed that Lignocaine significantly prolonged the duration of analgesia postoperatively (Table 4, Figure 4).
|
Time in minutes for rescue analgesia |
Group |
N |
Mean |
Std. Deviation |
T |
p value |
|
First rescue analgesia |
Control |
18 |
10.28 |
9.151 |
8.36 |
0.000 |
|
Lignocaine |
6 |
270.00 |
136.638 |
|||
|
Second rescue analgesia |
Control |
18 |
267.78 |
132.201 |
||
|
Lignocaine |
0a (was not required) |
. |
. |
|||
|
Third rescue analgesia |
Control |
8 |
581.25 |
127.776 |
||
|
Lignocaine |
0a (was not required) |
. |
. |
|||
|
a. t cannot be computed because at least one of the groups is empty. |
|
|||||
Table 4 Comparison of time for rescue analgesia (in minutes) in the two groups
Analysis of total amount of rescue analgesia in 24 hours in lignocaine and control group showed a significant statistical difference (p < 0.001). The amount of rescue analgesia required in lignocaine group was significantly less compared to the control group (Table 5, Figure 5).
|
|
Group |
n |
Mean |
Std. Deviation |
t |
p value |
|
Total amount of rescue analgesia in 24hr (in mg) |
Control |
18 |
199.44 |
19.84 |
13.474 |
0.001 |
|
Lignocaine |
18 |
33.33 |
48.507 |
Table 5 Comparison of total rescue analgesic requirement in 24 hours in the two groups
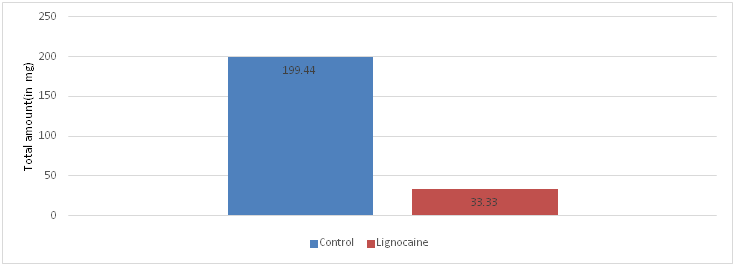
Figure 5 Graphical representation of comparison of total amount of rescue analgesia in 24 hours in lignocaine and control group.
Comparison of Postoperative heart rate between Lignocaine and control groups at definite time intervals postoperatively showed no statistically significant differences (p value > 0.05) (Table 6, Figure 6).
|
Adverse effect |
Control group |
Lignocaine group |
|
Nausea |
no |
no |
|
Vomiting |
no |
no |
|
Respiratory depression |
no |
no |
|
CNS toxicity signs |
no |
no |
|
CVS toxicity signs |
no |
no |
Table 6 Table showing comparison of adverse effects in control and lignocaine group
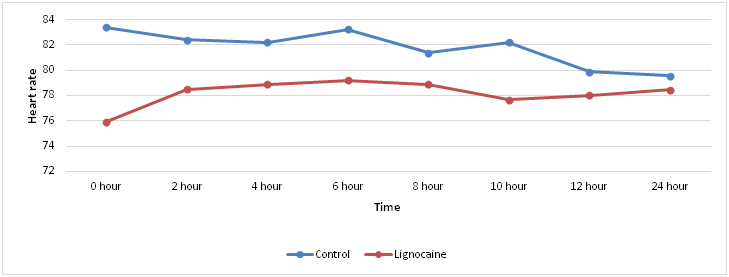
Figure 6 Graphical representation of postoperative heart rate at definite time intervals in the two groups.
Figure 7 Graphical representation of postoperative Mean arterial pressure in mm Hg at definite time intervals in both groups.
Analysis and comparison of postoperative Mean arterial pressure in mm Hg at 0hour, 2nd hour, 4th hour, 6th hour, 8th hour,10th hour, 12th hour and 24th hour postoperatively was also statistically insignificant (p value > 0.05) at all time periods in control and lignocaine group.
Adverse effects
The side effects looked for were nausea, vomiting, respiratory depression and signs of central nervous system or cardiovascular system toxicity of lignocaine. No adverse effects were noted in either Lignocaine or control group.
Laparoscopic cholecystectomy is the first line surgical treatment for symptomatic cholelithiasis and acute cholecystitis due to a number of advantages over open cholecystectomy, including decrease in duration of postoperative hospital stay.8 It is done as an ambulatory surgery but postoperative pain can cause delayed discharge from the day care unit. Optimal postoperative pain relief is not only needed for the patients’ comfort and satisfaction but also to facilitate their early mobilization and rehabilitation. Perioperative intravenous lignocaine is found to decrease postoperative pain and morphine requirements after prostatic surgery and colon surgery.9 It is also found to improve gut motility and decrease the length of hospital stay in laparoscopic colorectal surgeries.10 It has been found to decrease postoperative opioid requirements. Ease of availability, inexpensiveness, simplicity of administration and safety makes lignocaine infusion an attractive alternative choice for nonopioid analgesia.
Demographic data analysis of the study population on the basis of age, sex, weight, height, ASA physical status and anaesthesia duration revealed that there is no statistically significant difference between the two groups and were comparable in terms of age, sex, weight, height, ASA physical status and anaesthesia duration. Postoperative pain was assessed in our study at 0 (at the time when patient is shifted to PACU), 2nd hr, 4th hr, 6th hr, 8th hr, 10th hr, 12th hr and 24th hour using Numerical Rating Scale (NRS). The mean NRS score in the two groups over 24 hours and the mean NRS score at 0,2nd,4th,6th,8th,10th,12th and 24th hours postoperatively were analysed using independent t test and Chi-square test respectively and showed statistically significant difference between the two groups. Reduction in postoperative pain has reduced the postoperative opioid analgesic requirements and the associated complications of opioid analgesia. This reduction in postoperative pain as shown by decreased NRS score has translated to better patient comfort and early recovery. In lignocaine group only 6 subjects required first rescue agent whereas in control group all 18 patients required first rescue analgesia. None of the subjects in lignocaine group required any kind of rescue agents subsequently, where as 18patients and 8 in control group required rescue agents subsequently. The number of subjects requiring first rescue analgesia was compared with Fischer’s exact test and got a p value of 0.03. Analgesic requirements were reduced by lignocaine infusion in a statistically significant number of patients (only 6 out of 18 in study group). The analgesic effects produced by lignocaine was good in quality as evidenced by the absence of subjects of lignocaine group requiring any other rescue agent.
Rescue analgesic was given when the patient had a NRS score ≥ 4, the time of demand of rescue analgesia was noted in minutes. The mean time for first rescue analgesia in lignocaine group was 270 minutes where as in control group rescue analgesia was requested at a mean time of 10.28 minutes. This was analysed with independent t test and found to be significant p =0.00. Patients in lignocaine group did not require second and third rescue analgesic whereas in control group second rescue analgesic was requested at 267 minutes and third rescue analgesic was requested at 581 minutes. Hence perioperative lignocaine infusion extended good quality analgesia till 270 minutes in 6 out of 18 patients and in rest of the patients (12) there was good quality analgesia till end of 24 hours.
Patients with a NRS score ≥ 4 was given rescue analgesia. The first rescue agent given was Inj. tramadol 100mg given intravenously and if the patient was not comfortable in 10 to 15 minutes, a second rescue agent of Injection pentazocine 0.4mg/kg was given intravenously. Total amount of rescue agents over 24 hours was calculated and analysed with independent t test. Patients in lignocaine group required only 33.3mg of tramadol and did not required pentazocine at all whereas in control group over 24 hours mean tramadol requirement was 227 mg and 4.72 mg of pentazocine. Comparison of amount of rescue agents over 24 hours was found to be statistically significant, p value = 0.00. This proves that quality of analgesia provided by lignocaine is excellent. This has helped in early ambulation and early discharge from hospital.
Heart rate and mean arterial pressure were measured at 0,2nd,4th,6th,8th,10th,12th and 24th hours postoperatively in both groups and compared with independent t test. There was no statistically significant difference in both groups at all times. Heart rate and mean arterial pressure have increased in subjects of control group during complaints of pain as a physiological response compared to the individual values in subjects in lignocaine group but values were within the normal range of fluctuation (± 20% baseline) as the patients were immediately supplemented with rescue analgesia, hence no significant statistical difference was produced.
No side effects were noted in any group and no signs of systemic toxicity was noted in lignocaine group. We didn't measure the plasma level of lignocaine, hence the infusion dose was carefully fixed at 2mg/kg/hr.11 The side effects looked for were nausea, vomiting, respiratory depression and signs of central nervous system or cardiovascular system toxicity of lignocaine. No adverse effects were noted in either lignocaine or control group.
Perioperative intravenous infusion of preservative free lignocaine significantly reduces postoperative pain and opioid requirements in laparoscopic cholecystectomy. Hence intraoperative infusion of lignocaine is a useful adjunct in multimodal analgesia in laparoscopic cholecystectomy. 2mg/kg/hr infusion can be safely administered without checking the plasma level of the drug.

©2021 Augustine, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.