Journal of
eISSN: 2373-6437


Research Article Volume 15 Issue 4
1Department of Anesthesiology and Reanimation, University of Health Sciences, Ahi Evren Thoracic and Cardiovascular Surgery Training and Research Hospital, Türkiye
2Department of Anesthesiology and Reanimation, Karadeniz Technical University Faculty of Medicine, Türkiye
Correspondence: Ahmet Eroğlu, Department of Anesthesiology and Reanimation, Karadeniz Technical University Faculty of Medicine, Trabzon, Türkiye
Received: July 31, 2023 | Published: August 10, 2023
Citation: Topaloğlu SF, Eroğlu M, Çekiç B, et al. Comparison of patient-controlled epidural and intravenous analgesia methods for postoperative pain control in patients after hip surgery. J Anesth Crit Care Open Access. 2023;15(4):114-118. DOI: 10.15406/jaccoa.2023.15.00564
Objective: Patients undergoing total hip arthroplasty surgery are usually older and have limited cardiac and pulmonary reserves. Effective postoperative pain control with patient-controlled analgesia (PCA) may contribute to recovery in these patients. In this study, we aimed to compare the effectiveness of patient-controlled epidural analgesia (PCEA) and patient-controlled intravenous analgesia (PCIA) for pain control after hip arthroplasty.
Methods: Our study was carried out in the orthopedic operating room of our hospital on 50 patients undergoing elective hip arthroplasty. At the end of the operation, the patients (n=50) were randomly divided into two groups: Group epidural (Group E) and Group intravenous (Group IV)] using a double-blind selection method, and PCA protocol was started. During the determined follow-up periods, the patients were evaluated regarding hemodynamic data, pain and sedation levels, and possible side effects.
Results: In our study, no difference was observed between demographic data, heart rate, respiratory rate and mean blood pressure values in comparisons between two groups. In Group IV compared to Group E, visual analog scale (VAS) values (P<0.001), additional analgesic consumption (P<0.05) and undesirable effects such as nausea-vomiting and sedation (P<0.05) which were observed in postoperative pain follow-up were statistically significantly higher.
Conclusion: Our study concluded that for postoperative pain management, the PCEA method has better analgesic performance, improves pain control and reduces the occurrence of side effects in hip arthroplasty compared to the PCIA method.
Keywords: Postoperative pain, analgesia, patient-controlled, analgesia, epidural, Orthopedic Anesthesia.
Total hip arthroplasty causes severe pain. Patients undergoing this surgery are usually older and have limited cardiac and pulmonary reserves. The increased sensitivity to drugs and side effects in this age group makes it even more important to determine the postoperative analgesia method.1 The pain level is complicated to assess in the elderly, and patient-controlled techniques that allow self-administration of analgesics can solve this problem. Effective postoperative patient-controlled analgesia (PCA) can reduce harmful physiological responses and contribute to improved patient outcomes.2 Postoperative patient-controlled analgesia can be achieved by intravenous analgesia (PCIA) using opioids alone or by epidural analgesia in combination with a local anesthetic (patient-controlled epidural analgesia; PCEA). These techniques constitute the most significant recent advance in managing postoperative pain.3,4
The principle of PCA is that as long as the patient is in pain, it is a system in which the patient can self-administer the drug at the dose pre-programmed by the physician. PCA aims to keep the concentration of the relevant drug in the plasma at a constant level, provide effective analgesia with less drug dose and fewer side effects, and thus enable the patient to regain physical activity faster.
There is limited experience in the efficacy and safety of PCEA after total hip arthroplasty.5 Due to individual differences in pain experience and variability in responses to analgesic drugs, PCA may be a practical method for postoperative pain relief after total hip arthroplasty.
In this study, we compared the efficacy of PCEA and PCIA for pain control after hip arthroplasty. We also wanted to determine if using PCEA could reduce the number of opioids and their side effects.
Our study was carried out in the orthopedic operating room at the Medical Faculty Farabi Hospital operating room. After ethics committee approval, it was performed on 50 ASA (American Society of Anesthesiologists) I-III patients and 40-80 years old who would undergo elective hip arthroplasty, included in a randomized double-blind plan, and informed consent was obtained. Patients with coagulopathy and bleeding disorders, increased intracranial pressure, a history of opioid allergy, unable to communicate about using the PCA device, and previously diagnosed with the neurological disease were excluded from the study. Before the operation, patients were visited in the ward, standard evaluation was performed before anesthesia, information was given about the anesthesia method and postoperative pain control, and written consent was obtained.
After intravenous (IV) vascular access to the patients who were taken to the operating room, 20 ml/kg of crystalloid fluid replacement was performed, and heart rate (HR), noninvasive blood pressure (NIBP) and peripheral oxygen saturation (SpO2) were monitored as standard. For premedication, all patients were administered 0.05 mg/kg IV midazolam before the procedure. For spinal-epidural combined anesthesia, the patient was placed in a lateral position, and the L3-4 or L4-5 range was examined; the appropriate interval for the intervention was determined, and the area was cleaned with an antiseptic solution and covered with a sterile drape. After applying local anesthesia to the area to be operated on, the epidural space was entered with an 18 G epidural needle and hanging drop method. After entering the epidural space, the duramater was passed through the needle with a 26 G spinal needle, and the subarachnoid space was entered; 2 ml of 0.5% hyperbaric bupivacaine was given. Subsequently, the spinal needle was removed, and a 20 G catheter was inserted through the epidural needle into the epidural space (2-3 cm in the epidural space) and fixed. After the procedure, the patient is placed back in the supine position and the level of the sensory block is determined by a pinprick test (pinprick test is to determine the level of the sensory block with a needle for a painful invasive stimulus or a cotton piece for noninvasive tactile sensation and cold sensation determination), and motor block level is determined by Modified Bromage Scale (0: no motor block, 1: cannot lift the leg straight, foot and knee are mobile, 2: cannot bend the knee, only raises the foot, 3: complete paralysis). After achieving the desired motor and sensory block level, the surgery was started by giving the appropriate position. Until the end of the operation, if the patient felt pain, 0.5% isobaric bupivacaine was mixed with 5 ml + 5 ml saline, and 10 ml was administered through the epidural space.
At the end of the operation, the patients (n=50) were randomly divided into two groups, Group epidural (Group E) and Group intravenous (Group IV)] by double-blind selection method, and the PCA protocol was started. Group E (n=25) was determined as the group to be administered with the epidural method by PCA (Abbott Pain Management Provider, Abbott Laboratories USA) and Group IV (n=25) as the group to be administered by the intravenous method with PCA. The independent doctor prepared the PCA protocol in the anesthesia waiting room in the operating room, and the PCA solution was prepared as 180 ml with saline and 5 µg/ml fentanyl. After placing the prepared PCA solutions into the device, the bolus dose of 5 ml was adjusted to a 20-minute lock time and a 4-hour limit of 30 ml. At the end of the operation, the patients were reminded how to use the PCA device, which was mounted to the epidural catheter or the IV line with an entraining fluid.
In the postoperative 30th minute, 1st, 2nd, 4th, 8th, 12th and 24th hour follow-ups of the patients who came to the post-anesthesia recovery room at the end of the surgery and had a PCA device inserted into the epidural catheter or IV vascular access, respiratory rate (RR), heart rate (HR), noninvasive blood pressure (NIBP) values were recorded by the doctor and nurse who were unaware of the study. A measured systolic blood pressure of <80 mm Hg or a 30% decrease compared to baseline was considered hypotension. Hypotension was treated with IV 500 ml of Ringer's lactate fluid, and if fluid therapy was insufficient, IV 10 mg of ephedrine (Ephedrine Ampoule 2ml Biosel). Heart rate >120/min. was considered tachycardia, <50/min. was considered bradycardia. Sedation levels of the patients were evaluated with a 4-point scale during the same follow-up periods (0: fully awake, 1: slightly sedated, awakened by vocalization, 2: moderately sedated, awakened by touch, 3: deeply sedated, awakened by painful stimulation). The patients were sent to the ward when the Aldrete recovery score was ≥9.6
In all postoperative follow-up periods, the patients' pain assessments were measured using a visual analog scale (VAS is evaluated between 0-10. 0: no pain, 10: severe unbearable pain). The period from when the PCA device was inserted into the patient at the end of the operation until the first time the patient felt pain was defined as the time for the first analgesic requirement. When the VAS was above 3, it was considered insufficient analgesia and paracetamol 1gr IV was given as an additional analgesic. Possible side effects (sedation, respiratory depression, nausea and vomiting, pruritus, and hypotension) were evaluated in the same follow-up periods.
Statistical analysis
In the statistical evaluation, the data obtained from the research were evaluated with the help of the statistical package program in line with the objectives. Kolmogorov – Smirnov distribution test was used to examine the normal distribution. Non-parametric analyzes were applied when the assumption of normal distribution could not be achieved. Pearson’s chi-square test was used to compare qualitative data. The Mann-Whitney U test was used for comparing quantitative data in the case of two groups and for comparing parameters between groups. Data obtained by measurement are shown as arithmetic mean ± standard deviation, and data obtained by counting are shown as numbers (%). Wilcoxon signed-rank test was used for the intragroup comparisons of the parameters, and analysis of variance was used for repeated measurements. In the study, all findings were evaluated bilaterally at p=0.05 significance level and p=0.01 advanced significance level.
There was no statistically significant difference between the groups in terms of age, gender, ASA and body mass index (BMI), surgery time, and distribution of additional anesthetic drugs given through the intraoperative epidural catheter (P>0.05) (Table 1).
|
Group E |
Group IV |
p-value |
|
|
Age (Years) |
61.88±11.27 |
56.28±10.53 |
0.76 |
|
Female: n (%) |
17 (% 68 ) |
20 (% 80) |
|
|
Male: n (%) |
8 (% 32) |
5 (% 20) |
|
|
ASA I : n (%) |
8 (%50) |
8 (%50) |
|
|
II: n (%) |
17 (%50) |
17 (%50) |
|
|
BMI [kg/height (m)2] |
28.08±3.67 |
29.44±4.38 |
0.241 |
|
Surgery Time (min.) |
117.80±35.09 |
126.20±48.76 |
0.488 |
|
Patient in need of additional epidural LA during the operation |
|||
|
Yes: n (%) |
9 (%47,4) |
10 (%52,6) |
|
|
No: n (%) |
16 (%51,6) |
15 (%49,4) |
|
Table 1 Demographic Data of the Patients
There was no statistically significant difference between the groups regarding heart rate values and respiratory rate (P>0.05). The mean blood pressure values of the patients in all follow-up periods were similar between the groups (P>0.05) (Table 2). When the VAS value between the groups was compared, postoperative 1st, 2nd, 4th, 8th, 12th and 24th VAS values were significantly higher in Group IV compared to Group E (P<0.001) (Figure 1).
|
Follow-up period |
Group E |
Group IV |
p-value |
|
Baseline |
95,20±10,51 |
6,00±10,24 |
0.827 |
|
Postop. 30th min |
83,60±11,12 |
84,00±9,90 |
0.468 |
|
Postop. 1st h |
85,40±6,30 |
85,20±8,45 |
0.764 |
|
Postop. 2nd h |
84,60±8,43 |
87,80±8,42 |
0.702 |
|
Postop. 4th h |
86,80±8,94 |
86,80±10,62 |
0.703 |
|
Postop. 8th h |
86,80±9,59 |
89,60±8,34 |
0.412 |
|
Postop. 12th h |
85,40±8,03 |
88,80±9,44 |
0.434 |
|
Postop. 24th h |
84,40±7,88 |
89,80±7,27, |
0.213 |
Table 2 Mean Blood Pressure Changes of the Patients (mm/Hg)

Figure 1 Visual Analogue Scale (VAS) Periodic Change of Groups.
*P<0.01 Significance level relative to Group E.
(T0, Baseline; Postoperative T1, 30th min; , T2, 1st hour; T3, 2nd hour; T4, 4th hour; T5, 8th hour; T6, 12th hour; T7, 24th hour).
In the postoperative pain treatment of the groups, additional analgesic consumption (paracetamol; 1gr) was used in bolus analgesic management with PCA in cases where VAS>3 was significantly higher in Group IV (0,64±0,81) than Group E (0,16±0,37) (P<0.05) (Figure 2).
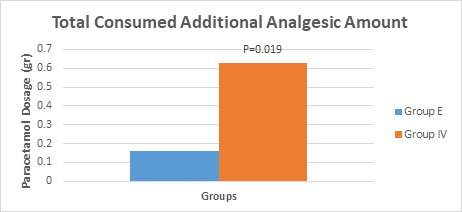
Figure 2 Postoperative Additional Analgesic Consumption Values of the Groups.
P<0.05 Significance level relative to the epidural group.
Comparing the groups, the number of PCA patient drug demand was significantly higher in Group IV compared to Group E at the 30th minute, 1st, 2nd, 4th, 8th, 12th and 24th hour follow-up periods (Figure 3).

Figure 3 Postoperative PCA Drug Demand Frequency of the Groups.
* P<0.01 degree of significance relative to the epidural group.
#P<0.05 degree of significance relative to the epidural group.
(Postoperative T1, 30th min; T2, 1st hour; T3, 2nd hour; T4, 4th hour; T5, 8th hour; T6, 12th hour; T7, 24th hour).
The frequency of total bolus drug demand from the PCA device and bolus drug administration was significantly higher in Group IV compared to Group E (P; 0.0001) (Figure 4). While approximately 575 µg fentanyl was used in Group IV, approximately 325 µg fentanyl was used in Group E. Total bolus drug demand/administration rates from the PCA device were similar between the groups (P=0.262) (Figure 4).

Figure 4 Postoperative PCA Drug Total Demand and Administration Number and Rate Values of the Groups.
P<0.01 degree of significance relative to the epidural group.
During the study, side effects such as respiratory depression, pruritus, and hypotension were not observed in either group. Nausea-vomiting was observed at a higher rate in Group IV than in Group E; only the postoperative 1st hour was statistically significant (P<0.05) (Table 3). When the sedation score between the groups was compared, postoperative T1=30th min.; T2 = 1st hour; T3=2nd hour; T4=4th hours, and T5=8th hour sedation levels were higher in Group IV than Group E. (T1, T2, T3, T4, T5, respectively; P;0.021; 0.0001; 0.010; 0.0001; 0.024, respectively) (Figure 5).
In our study, we found that the PCEA method was superior to the PCIA method in the treatment of postoperative pain in patients who had undergone total hip arthroplasty, and adverse effects such as nausea and vomiting, and sedation were significantly less observed in the PCEA method. Considering the PCA pain control method as a safe and effective management option, although there are intravenous, epidural and subcutaneous administration routes, this technique can be performed as infusion, bolus or infusion + bolus administration.
In the PCA method, it has been shown that intermittent bolus analgesia administration instead of continuous infusion is a safe method that provides patient comfort in the postoperative period and can be preferred due to advantages such as fewer side effects, less drug consumption and effectiveness in pain control.7,8 We also preferred the PCA intermittent bolus method in our study. With this method, while the hemodynamic data of the patients remained stable in the postoperative period, patients reported VAS scores similar to previous studies. In addition, we observed a lower frequency of side effects such as nausea, vomiting and sedation with less drug consumption.
Intravenous PCA is a widely used postoperative analgesic strategy because of its efficacy and safety as an acute postoperative pain reliever (3). Regarding side effects, it has been found to provide significant advantages compared to other postoperative analgesia applications, such as traditional intravenous, oral, and patient-controlled transdermal administration.9-12 However, epidural PCA causes less drug consumption, easier patient follow-up, effective analgesia, and fewer side effects in appropriately selected patients.13,14
Today, studies are comparing both methods in terms of providing effective analgesia in different surgeries.15-18 These studies demonstrated that PCEA provides more significant benefits than PCIA in postoperative analgesia and can effectively relieve pain with significantly lower VAS scores after some major spine surgery, hip surgery, and gynecologic surgery. In our study, we observed lower VAS scores in patients who underwent PCEA compared to patients who underwent PCIA, and we concluded that the epidural group was more effective in postoperative pain control.
In addition to low VAS scores in postoperative pain treatment, the amount of additional analgesic consumption is an important parameter in effective pain management. In the study of Sang Hoon Lee et al.,17 in which they compared the efficacy of epidural and intravenous PCA in treating pain after posterior lumbar surgery, they found the need for additional analgesics to be significantly higher in the intravenous group compared to the epidural group. Our study found that analgesic consumption was significantly higher in the intravenous group compared to the epidural group. Accordingly, we observed that the number of PCA drug demand and the number of drug administration was higher in the IV group at all follow-up hours. PCA drug selection may vary depending on the method applied. In most studies in the literature, local anesthetic and opioid combinations are primarily preferred in order to avoid possible side effects of opioids when comparing epidural and IV methods.15-18 We used opioids (fentanyl) in both groups and did not observe side effects such as respiratory depression and pruritus. However, in the intravenous group compared to the epidural group; we observed more nausea and vomiting in the postoperative 1st hour and a higher sedation score in the postoperative first 8 hours. We think that more nausea-vomiting and sedation in the intravenous group are due to the demand and administration of more drugs in the IV group.
Our study concluded that for postoperative pain management, the PCEA method has better analgesic performance, improves pain control and reduces the occurrence of side effects in hip arthroplasty compared to the PCIA method.
The authors have no conflict of interest to declare.
The authors declare that this study had received no financial support.

©2023 Topaloğlu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.