Journal of
eISSN: 2373-6437


Review Article Volume 6 Issue 4
Consultant Intensive Care Medicine at Private practice clinic, Iraq
Correspondence: Fadhil Zwer, Consultant Intensive Care Medicine at Private practice clinic, Iraq
Received: January 25, 2016 | Published: December 21, 2016
Citation: Zwer F (2016) Biomarkers of ALI/ARDS. J Anesth Crit Care Open Access 6(4): 00237. DOI: 10.15406/jaccoa.2016.06.00237
The validation of biomarkers, for use in clinical trials and ultimately in practice, has become a central tenet of translational biomedical research.1 Biomarkers are potentially useful as guides to clinical management and as research tools. In the clinical setting there is a high valuable on biomarker data being easily and safely obtained within a timeframe that is relevant to the disease process (Table 1). For example, an indicator of poor prognosis that may encourage referral to a specialist center would need to be available within hours, whereas a marker of ventilator-associated lung injury (VALI) that was being used to fine-tune ventilator settings would have to be turned around within minutes. No biomarkers that are currently available have covering routine clinical practice with the possible exception of the use of pro calcitonin to diagnose sepsis in critically ill patients and to guide their antibiotic therapy (Table 1).1
Measurement is safe and feasible in the critically ill. |
Sensitive, reproducible and specific. |
Timely. |
Modified by an effective intervention to change the target outcome of interest. |
Table 1 Proposed characteristics of an ideal biomarker for acute lung injury
The use of biomarkers to refine patient populations, such that clinical trials will be most likely to provide a definitive answer requiring the fewest patients, is particularly appealing for application to research involving patients with a heterogeneous syndrome like ALI/ARDS. This could be beneficial by helping to characterize either a group of patients with a high mortality where mortality is the primary outcome measure, or by identifying patients in whom a pathological process, which is targeted by an intervention, is particularly prominent.
Proposed criteria for characterizing ideal biomarkers for ALI, most of which are self explanatory are listed in. It has been argued that biomarkers should inform or, at least, relate to the disease pathogenesis. Why confuse explaining mechanisms with the pragmatic business of identifying biomarkers? We prefer as wide a definition as possible; for example, the electrocardiograph has been one of the most useful biomarkers in medicine but not much can be learned about the pathogenesis of myocardial infarction through its study.
The current definition of ALI/ARDS is such that biomarkers of the established syndrome are largely redundant. An exception would be a biomarker that was specific to the pathological process described as diffuse alveolar damage. That is, a biomarker that could exclude patients from studies who fulfilled the diagnostic criteria but who essentially have a distinct disease, which may have a different natural history and specific treatment, for example, cardiogenic pulmonary edema, eosinophilic pneumonia and pulmonary embolism. Most studies have attempted to correlate selected biomarkers with disease severity or death, which is potentially useful both clinically to help target resources and more expensive or invasive management strategies, and in helping to power research studies using mortality as the primary outcome.
It is proposed that the use of biomarkers in a complex syndrome like ALI/ARDS is most likely to be effective when they are specific to an individual component or process that can be manipulated. One productive approach has been to measure plasma and bronchoalveolar lavage [BAL] fluid levels of mediators as a reflection of systemic and pulmonary inflammation respectively. In samples from large multicenter trials elevated levels of mediators, like soluble tumor necrosis factor-alpha receptors (sTNFR) 1 and 2, soluble intercellular adhesion molecule-1 and interleukin (IL)-6 were associated with adverse outcomes in patients with ALI/ARDS. The limitations of this strategy are that these mediators have multiple effects, have no specificity to the lung and there is no convincing evidence that manipulating the inflammatory response benefits patients with ALI/ARDS.
Partly because of the realization that ventilator-associated lung injury plays a major part in the pathogenesis of ALI/ARDS and, as a result, many large studies have been performed examining the effects of ventilator strategies, a lot has been learned about the responses of popular biomarkers in patients undergoing protective and standard ventilation. Hence, circulating mediators of inflammation (sTNFR, IL-6, -8 and -10), indicators of epithelial cell injury (soluble advanced glycation end-product receptors (sRAGE)) and surfactant protein-D) and components of the coagulation system (protein-C and plasminogen activator inhibitor-1) have all been promoted as biomarkers of ventilator-associated lung injury.
However, because the proposed mechanism, whereby ventilator-associated lung injury kills patients through the exacerbation of local injury and inflammation, the mediators of which then leak into the systemic circulation causing multiple organ dysfunction, it would be surprising if there was not considerable overlap between markers of ventilator-associated lung injury, tissue injury, inflammation and a poor prognosis. In other words, these biomarkers inevitably lack specificity for individual processes or outcomes.2
The relationship of ALI/ARDS biomarkers and its pathogenesis
Over the past two decades focus on biomarkers in ALI/ARDS has yielded important information regarding the pathophysiology of the lung injury and repair and have highlighted what cells and their mediators have been involved. Studies of biomarkers have also in directly led to the generation of new ideas regarding potential novel therapeutic targets. These biomarkers in ALI/ARDS can reflect either cellular activation or cell injury, as well as ongoing acute activation of the inflammatory, coagulation and fibrinolytic systems. Some of the biological markers may possess pleiotropic effects and may have a role in the repair process. Some of these biomarkers have been investigated as potential surrogate markers for the development of ALI/ARDS as well as clinical outcomes, such as ventilator free days, morbidity and mortality. Currently, biomarkers in ALI/ARDS remain primarily within the domain of a research tool to aid in the delineation of the patho physiologic mechanisms involved in acute lung injury and its subsequent repair although they have been shown to have prognostic value as well. The validation of readily measurable biomarkers that may have a role in the design of future clinical trials or in selecting subgroups of patients with ALI is an important longer term objective. In addition to clinical criteria for ALI, biomarkers could be of value in the evaluation and testing of new pharmacologic or cell-based therapies for ALI/ARDS.
Inflammatory factors: The inflammatory responses in ALI/ARDS can either be directly related to an ongoing primary infectious stimulus such as pneumonia or there may be systemic inflammation which is being amplified by the lung injury. The inflammatory cascade involves inflammatory cells and the release of inflammatory mediators. For instance, comparing the ratio of cytokines in serum compared to BAL fluid is suggesting that most of the inflammatory mediators have a pulmonary origin and not just solely the effect of the exudative phase of ALI/ARDS with the flooding of the alveolar environment with serum mediators. There are both pro inflammatory and anti-inflammatory mediators and therefore it maybe more the balance of these mediators and their biological inhibitors in the surrounding milieu that regulate much of the development of lung injury and repair which may have implications for their use as biomarkers in isolation or in combination. Most publications have focused on the significance of antigenic levels of inflammatory biomarkers rather than determination of the net inflammatory balance (use of molar ratios) which may be of greater physiological and clinical importance. Inflammatory mediators have been measured both in the plasma or serum, and locally in bronchoalveolar lavage fluid or undiluted pulmonary edema fluid. These mediators may be actively secreted from cells which have been recruited into the air spaces in response to the inflammatory cascade or may appear due to release from cellular death. These inflammatory mediators include the pro inflammatory cytokines interleukins (IL) such as IL-1β, TNFα, IL-6, and IL-8 which possess potent pro-inflammatory actions, as well as the anti-inflammatory interleukins including IL-1ra, IL-10 and IL-13 (Figure 1).
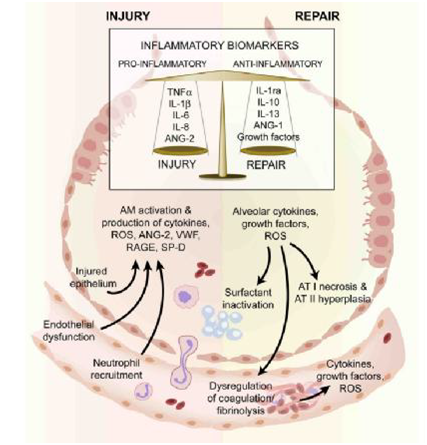
Figure 1 The injured alveolus in the acute phase (left hand side) and the repair phase in acute lung injury.
In the acute exudative phase, there is activation of resident alveolar macrophages which results in production of several pro-inflammatory molecules. These stimulate chemotaxis and activation of neutrophils which release a variety of mediators that further increase the pro-inflammatory environment of the injured alveolus and are associated with both alveolar endothelial and epithelial injury. Some of the mechanisms that play a role repair in ALI is illustrated (right hand side). Alveolar type II cells undergo hyperplasia and there is also recruitment of fibroblasts (not shown). There is release of growth factors and anti-inflammatory cytokines involved in repair.
These mediators as well as cell specific activation/injury can be measured as biomarkers. AM–alveolar macrophage; ANG–angiopoietin; AT–alveolar epithelial cell type; IL–interleukin; IL-1ra –interleukin 1 receptor antagonist, RAGE–receptor for advanced glycation end products, reactive oxygen species, SP=D–surfactant D; VWF–von Willebrand factor.3
One of the most biologically active cytokines in the early phases of ALI/ARDS is IL-1β, which is elevated in plasma and is predictive of clinical outcomes. IL-1β is a potent inducer of lung fibrosis and causes release of a variety of pro-inflammatory chemokine e.g. monocyte chemotatic protein (MCP)-1, macrophage inflammatory protein (MIP) -1α, IL-6and IL-8 with subsequent recruitment of inflammatory cells into the air spaces as well as being able to alter endothelial-epithelial barrier permeability and fluid transport leading to edema (which is mediated in part via the αvβ5/β6 integrins pathway). IL-1β is elevated in plasma and BAL fluid in patients with ALI/ARDS, but also is elevated in higher tidal volume and lower positive end expiratory pressure ventilation. Due to these important clinical findings in patients with ALI/ARDS and the understanding of IL-1 biology including the knowledge of the existence of the presence of the naturally occurring inhibitors of IL-1signalling namely IL-1Ra or sIL-1RII, it is proposed that these could be putative novel therapeutic targets due to their potential to possess anti inflammatory activities. Another early pro-inflammatory cytokine which is in both plasma and BAL fluid in the early phase of ALI is TNFα. It is postulated that resident alveolar macrophages stimulated by pathogen recognition generate much of the production of the early cytokines, IL-1β and TNF-α, which in turn stimulate neighboring cells to produce a battery of chemokines that mediate the recruitment of neutrophils, monocytes and lymphocytes into the alveolar space. The alveolar macrophages may initially be important therefore in the pro inflammatory stage but they also have been postulated to have important anti-inflammatory activity in there solution/repair phase via phagocytosis of apoptotic neutrophils and cell debris as well as secreting epithelial growth factors in a TNFα dependent mechanism. TNFα also has been demonstrated to indirectly promote pulmonary edema by producing reactive oxygen species which subsequently decrease the expression of ENaC and the Na+-K+-ATPase. IL-6 and IL-8 are two other pro-inflammatory cytokines which have been demonstrated to be elevated in both plasma and BAL fluid and are predictive of poor outcomes in ALI/ARDS patients. IL-8 has a role in neutrophil and monocyte chemotaxis and inhibits neutrophil apoptosis and elevated levels correlates well with number of neutrophils and total protein (a surrogate marker for permeability of the alveolar barrier) in BAL fluid and also are elevated in non-survivors compared to survivors in single center and multi-center studies. IL-6 cytokine is involved in ALI/ARDS that signals through the ERK pathway and can activate multiple signal transduction pathways including Janus kinase/signal transducer and activator of transcription (JAK/STAT), Rat sarcoma/extracellular signal-regulated kinase (Ras/ERK) and the PI3-K/Akt. IL-6 is critical for B-cell differentiation and maturation with secretion of immunoglobulins, cytotoxic T cell differentiation, macrophage and monocyte function and production of acute phase proteins. Although IL-6 activates both pro inflammatory and anti inflammatory mechanisms, IL-6 primarily has a pro-inflammatory profile. The presence of the IL-6 agonist sIL-6R has also been investigated in ALI/ARDS and higher molar ratio ofIL-6/sIL-6R is associated with a higher risk of death.
It had been found that the activation of the inflammatory cascade results in the activation of the coagulation system which in turn can influence inflammatory responses by effecting expression of IL-1, IL-6 and IL-8 and migration of inflammatory cells across the endothelial and epithelial barriers into the alveoli. The pro-inflammatory events may also inhibit fibrinolysis and also induce platelet activation. Several coagulation biomarkers have been demonstrated to be abnormal in ALI/ARDS including protein C, plasminogen activator inhibitor (PAI-1) and thrombomodulin. Thromomodulin activates protein C leading to the formation of a thrombus. The alveolar epithelium contains thrombomodulin which can activate protein C leading to formation of activated protein C, an important endogenous anticoagulant. Activated protein C can improve endothelial permeability via activation of thesphigosine-1-phosphate pathway and suppression of pro-inflammatory cytokines. In ALI/ARDS, the plasma and BAL fluid levels of protein C (part of the activated protein C complex) are low and the plasma levels of PAI-1 are elevated, and both of these finding shave been associated with increased mortality. Therefore there was a rationale for drug trials in patients with ALI/ARDS with therapeutic interventions focused on administering pharmacologic doses of human recombinant activated protein C. Based on in vitro studies, activated protein C can protect the endothelial barrier via protease activated receptor-1 (PAR-1) dependent mechanisms.
Fibrinolytic activity is decreased in patients with ALI/ARDS which may in part be related to high BAL fluid levels of PAI-1 as well as decreased fibrinolysis there is also the occurrence of increased fibrin production. Endothelial cells, epithelial cells, macrophages, and fibroblasts. The significantly higher plasma levels of PAI-1 in ALI/ARDS patients who do not survive may reflect a greater impairment in the fibrinolytic system in these patients.
Platelets also play a crucial role in homeostasis and thrombosis as well as the coagulation and fibrinolytic system. However, over the last decade it has been documented that the platelets have a diverse and extended role via their ability to release mediators to recruit inflammatory cells and progenitor cells, release pro- and anti-inflammatory cytokines, as well angio genic factors, all of which may contribute to the pathobiology of ALI/ARDS. All of these pathways utilize a variety of active moieties including chemokines, P-selectins and other adhesion molecules as well as signalling molecules such as Src kinases, all of which may be future therapeutic targets.
Growth factors appear to play a major role in the repair and resolution of ALI/ARDS. The repair of the damaged alveolar epithelium is an incompletely understood process that involves hyperplasia of type II pneumocytes (and perhaps type I pneumocytes), migration along the basement membrane by the type II cells to form a new epithelial barrier, and complex interactions with extracellular matrix and other cells such as alveolar macrophages. A variety of growth factors promote repair of the alveolar epithelium including keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), epidermal growth factor (EGF),acidic fibroblast growth factor (FGF), retinoic acid, transforming growth factor-α(TGF-α)and Insulin like growth factor (IGF-1). Lung endothelial repair is promoted by vascular endothelial growth factor (VEGF). Repair can be accompanied by fibrosis that may be promoted by TGF-β, activin-A, platelet derived growth factor (PDGF), basic FGF, IGF-1 be promoted by TGF-β, activin-A, platelet derived growth factor (PDGF), basic FGF, IGF-1 but inhibited by HGF and Interferon-γ (IFN-γ). There are at least two major pathways that growth factors utilize in ALI/ARDS, either tyrosine kinase receptor mediation e.g. KGF, HGF, FGF, VEGF, EGF and PDGF, or serine-threonine kinase receptors such as TGFβ1which tend to have an opposing effect on the up regulation that occurs when the tyrosinekinase receptor pathway is utilized. Anyhow, some of these growth factors have been studied as biomarkers in ALI/ARDS.
KGF and HGF are potent mitogens for type II pneumocytes and are postulated to play an important role in the repair of the epithelium following lung injury. KGF and HGF are elevated in BAL fluid from patients with ALI/ARDS. HGF can be produced by alveolar neutrophils, macrophages, endothelial cells and fibroblasts and up regulated by a variety of pro-inflammatory cytokines such as IL-1β and TNFα via a COX2/PGE2 dependent mechanism. HGF can also be released by the proteolytic activity of proteases that cleave the HGF from the extracellular matrix. However, in the lung, VEGF is produced primarily by epithelial cells. VEGF seems to increase micro vascular permeability in ALI/ARDS, but also during the repair phase has an important role in increasing endothelial cell proliferation and survival. The levels of VEGF are increased in plasma from patients with ALI/ARDS, but are decreased in BAL fluid compared to healthy controls. Subsequently BAL fluid VEGF increases during the resolution of the lung injury.
Biomarkers of alveolar epithelial/endothelial injury: Because alveolar epithelial injury plays an important role in determining the severity of ALI/ARDS, investigators have studied biochemical markers that may reflect injury to either the type I or type II alveolar epithelial cell. Surfactant proteins are primarily secreted by type II pneumocytes and are which help maintain low alveolar surface tension preventing alveolar collapse and they have a role in the innate immune defense of the lung. Surfactant protein D is primarily a product of type II cells and the receptor for advanced glycation end-products (RAGE) is released primarily by type I cells. RAGE belongs to the immunoglobulin super family and binds calgranulin (also called ENRAGE) or high mobility group box-1(HMGB-1) which activates NFκβ leading to the production of pro-inflammatory cytokines, reactive oxygen species and protease production. Both of these proteins are elevated in the plasma in ALI/ARDS patients and have been associated with worse clinical outcomes. Elevated levels of plasma RAGE early in ALI/ARDS seem to identify patients with more alveolar epithelial injury and it is these patients who benefited most from low tidal volume ventilation in the randomized ARDS net trial. Another epithelial protein which has been studied as a potential biomarker is an epithelial mucin protein, called Kerbs von denLungren-6 (KL-6), which is unregulated during injury on the surface of the type II pneumocytes. KL-6 has been demonstrated to be increased in interstitial lung disease in which there is disarray of the alveolar structure and is correlated with increased barrier permeability. It is important to notice that an early pulmonary edema level of KL-6 has been demonstrated to be elevated in patients with ALI/ARDS who did not survive compared to patients with ALI/ARDS who have better mortality but is not able to predict those patients who are at risk of developing ALI. As well as the important role the alveolar epithelium type I and II play in acute lung injury and repair there is one other cell type which produces a protein which may have an important role as a biomarker, namely the Clara cell and the protein called Clara Cell specific protein (CC-16). Nevertheless, elevated CC-16 serum level was associated with increased risk of mortality.
It was found that not only is there epithelial injury but endothelial dysfunction also plays a central role in lung injury and repair (Figure 1) not only in contributing to the influx of inflammatory cells and mediators during the exudative phase but is an active source of growth factors and mediators that can affect vascular tone, cellular proliferation and angiogenesis. The endothelium is also the site in which the above systems of inflammation, coagulation and fibrinolysis cross talk can occur and therefore influences directly the outcome of these systems. Endothelial activity is detected again by the use of a variety of potential biomarker s including von Will brand factor (VWF), angiotens in converting enzyme or tissue factor pathway inhibitor and many studies have focused on these in acute lung injury as has been reviewed recently.4 VWF has been well documented to be increased in plasma and BALF of patients with acute lung injury as well as be associated with being predictive of outcome of these patients. VWF is a glycoprotein that is secreted by both the megakaryocytes and the vascular endothelium and has a pivotal role in homeostasis by being the carrier protein for clotting factors such as factor VIIII as well as acting as a mechanical bridging component between the platelets and the endothelium. However the patients in the early phase of ALI the pulmonary edema fluid and the plasma levels of VWF were associated with clinical outcome demonstrating that the endothelium activation is associated with increased mortality.3
Neutrophil elastase predictor for acute lung injury
Acute lung injury or acute respiratory distress syndrome is an acute inflammatory lung injury that may be associated with systemic inflammatory response syndrome (SIRS) and develops after a latent period of hours or days subsequent to various predisposing conditions, including multiple trauma, burns, and acute pancreatitis. Neutrophils and their products especially reactive oxygen species and serine proteases such as elastase, are implicated in the pathogenesis of ALI/ARDS. In fact, neutrophil elastase is elevated in the circulation of ARDS patients. In addition, it has been reported that sivelestat, a specific elastase inhibitor, is efficacious in a variety of animal models of ALI. These findings indicate that elastase could be a therapeutic target for the treatment of ALI/ARDS. However, the clinical trial of sivelestat failed to improve the survival rate of ALI/ARDS, although some beneficial effects such as reduced duration of mechanical ventilation were observed. In the previous studies, sivelestat was always administered after the development of ALI/ARDS, implying that the lung injury is already established before the administration of sivelestat. The failure of sivelestat to improve the survival rate of ALI/ARDS might be ascribed to the late administration of this inhibitor. The timing of any neutrophil elastase-directed intervention may be critically important for prevention of the development of ALI/ARDS. It is expected that early administration of an elastase inhibitor may provide a chance of success. For appropriate administration for appropriate patients, it is essential to determine the signs or factors predicting the imminent development of ALI/ARDS. A possible predicting factor may be the critical level of plasma neutrophil elastase, which may determine the development of ALI/ARDS in certain patients.
It had been found that the plasma neutrophil elastase level was significantly elevated in all patients with SIRS alone and ALI/ARDS with SIRS as compared with healthy controls. The elastase level of ALI/ARDS with SIRS was significantly greater than that of SIRS alone. The level of neutrophil elastase in all patients with SIRS alone was consistently less than 220ng/ml. By contrast, the maximal level of neutrophil elastase in most patients with ALI/ARDS with SIRS was more than 220ng/ml, and all patients with SIRS expressing the elastase level of more than 220ng/ml finally developed ALI/ARDS. These findings indicate that 220ng/ml is the critical level of plasma neutrophil elastase. The patients presenting SIRS with more than 220ng/ml neutrophil elastase are highly likely to develop ALI/ARDS, which develops within 2 days after the detection of more than 220ng/ml elastase in most patients. It was suggest that these patients may be a good candidates for early administration of elastase inhibitors to prevent the development of pulmonary dysfunction.
Although the level of neutrophil elastase in all patients with SIRS alone was consistently less than 220ng/ml but this level does not indicate the intact pulmonary function, and ALI/ARDS developed in some SIRS patients with less than 220ng/ml elastase.
It is possible that, in addition to neutrophil elastase, other factors such as reactive oxygen species may play an important role in the lung injury in these patients. In fact, a massive amount of superoxide is released from neutrophils by the adhesive interaction with endothelial cells stimulated by pro inflammatory cytokines (IL-1b and TNF-a). In addition, it has been reported that a significant part of active elastase is associated with the plasma membrane on activated neutrophils, which may contribute to the tissue injury in concert with reactive oxygen species. It is also possible that in some patients neutrophils are not primarily involved in the lung injury, as indicated by the findings that ALI/ARDS develops in certain patients with severe neutropenia.
Inflammatory cytokines such as IL-1b, IL-6, IL-8, and IL-10 were also elevated in the circulation of some patients with ALI/ARDS. However, no statistical significance in the levels of these cytokines was observed among the patients which, indicating that the level of these cytokines is not a reliable predicting factor for the development of pulmonary dysfunction or ALI/ARDS. Although it is possible that these cytokines, especially IL-1b and TNF-a, may play an important role in the development of lung injury, consistent elevation of these cytokines at the high level was not detected in the circulation, and the serum levels were not correlated with the severity of pulmonary dysfunction. No remarkable elevation of IL-1b and TNF-a in the circulation may be ascribed to rapid binding of these cytokines to a variety of cells, including endothelial cells and neutrophils, and degradation of these cytokines by neutrophil elastase.5 By contrast, neutrophil elastase forms the complex with protease inhibitors such as α1-proteinase inhibitor and α2-macroglobulin in the plasma, allowing the easy detection of the elevated level of neutrophil elastase. The pathogenesis and the underlying diseases of the patients with pulmonary dysfunction or ALI/ARDS are heterogeneous, and neutrophil elastase might not be a primary causative agent for the lung injury in some patients. The heterogeneity of the underlying diseases and the late administration of sivelestat may partly explain why the clinical trial of sivelestat in a large scale failed to improve the survival rate of ALI/ARDS. It is indicated that the critical level of plasma neutrophil elastase may be 220ng/ml, and the SIRS patients with more than 220ng/ml neutrophil elastase are highly likely to develop ALI/ARDS. In most patients, a level of greater than 220ng/ml of elastase and the development of ALI/ARDS were observed within 3 days after the accident. In addition, in most patients the level of more than 220ng/ml elastase was already detected when the diagnosis of ALI/ARDS with SIRS was made. These findings indicate that the sequential monitoring of plasma neutrophil elastase for at least 3 days after the accident may be useful for determination of the patients susceptible to the development of ALI/ARDS.6,7
Kerbs von Lundgren 6 antigen (KL-6) biomarker
KL-6 levels are elevated in plasma from patients with ARDS: KL-6 is a mucin-like glycoprotein expressed on epithelial cells. KL-6, a pulmonary epithelial mucin with low molecular weight, is an integral membrane glycoprotein classified as cluster 9 (MUC1), with an extracellular domain consisting mostly of tandem repeats of 20 amino acid sequences and a cytoplasmic tail. KL-6 splits off at the S-S bond near the epithelial membrane surface and becomes distributed in pulmonary epithelial lining fluid. Immunohistochemical studies have shown that KL-6 is strongly expressed on type II pneumocytes, and serum KL-6 levels are regarded as an index of alveolar epithelial cell damage and subsequent regeneration. Recently, it is reported that the localization of KL-6 protein appeared primarily on the apical surface of alveolar type II cells in post mortem ARDS patients (Figure 2).8 Serum levels of KL-6 are elevated in a variety of respiratory and non-respiratory conditions, including breast and pancreatic cancer and diabetes mellitus. However, most attention has focused on KL-6 as a diagnostic and prognostic tool in respiratory disease. Thus, serum KL-6 levels are elevated and correlate with disease activity in patients with interstitial pneumonia, alveolar proteinosis, pulmonary sarcoidosis and radiation pneumonitis. Moreover, serum KL-6 levels have been shown to correlate with indices of alveolar-capillary permeability, suggesting a link between serum KL-6 and alveolar epithelial barrier dysfunction. Damage to, and disruption of, the alveolar epithelial lining is a key feature in the pathophysiology of ARDS, and leads to the development of pulmonary edema and respiratory failure. Furthermore, inappropriate ventilatory strategies that lead to the cyclical opening and closing of at electatic alveoli, together with alveolar over-distention, can worsen pre-existing lung injury.
It was found that the alveolar barrier integrity to albumin was disrupted at the onset of ALI. However, despite the nearly 10-fold higher concentration of KL-6 in epithelial lining fluid of patients with ALI than in controls, the plasma levels at the onset of ALI were similar to controls, perhaps because of the relatively large molecular size of KL-6. The molecular mass of KL-6 antigen, which includes large amounts of saccharides, is estimated to be at least 1,000–2,000kDa, whereas that of albumin is 67kDa. This molecular size difference may explain the slower diffusion of KL-6 through the paracellular pathway of lung endothelium and alveolar epithelium compared with albumin (Figure 3). The extent of alveolar septal barrier injury responsible for the leakage of albumin into the alveolar lumen may not have been sufficient to cause significant alveolar KL-6 leakage into the bloodstream. In some patients, increased plasma KL-6 concentrations were present at the onset of ALI.
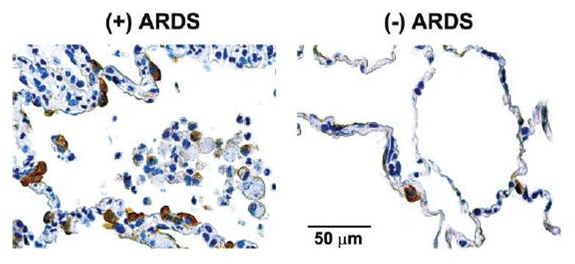
Figure 2 Representative immune histochemical staining for KL-6 in a postmortem lung specimen obtained from a patient who died with ALI [(+) ARDS] and from a patient who died of non pulmonary causes [(-) ARDS].
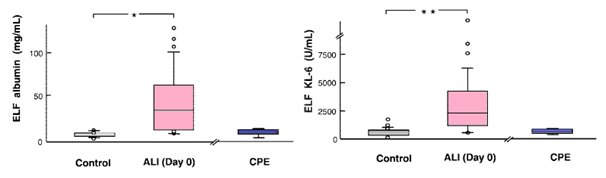
Figure 3 Albumin (top) and KL-6 concentrations (bottom) in epithelial lining fluid (ELF) in control patients, patients at the onset of acute lung injury with cardio genic pulmonary edema (CPE).
This early increase in plasma KL-6 concentration especially in non-survivors suggests that the alveolar barrier might be more disrupted in those patients. The significant increase in KL- 6 concentrations in ELF and plasma at the onset of ALI in non-survivors further supports the hypothesis that alveolar epithelial cell injury may be a crucial determinant of prognosis of ALI and that measurements of KL-6 might be useful to predict its outcome. However, from the operating characteristics curve analyses, KL-6 levels in both epithelial lining fluid and plasma were sensitive and specific markers of fatal outcomes.
KL-6 protein was predominantly expressed on the surface of epithelial cells (brown stain), probably type II cells. KL-6 was also expressed on apparently denuded epithelial cells in the alveolar space.8 The Box-whisker plots show the 25th and 75th percentiles, the median (horizontal line within the box), and the 10th and 90th percentiles (whiskers). *P _ 0.0001, **P _ 0.001 (ALI vs. control patients).8
It was demonstrated that there is an elevated levels of KL-6 in plasma from patients with ARDS when compared to ventilated controls of matched illness severity and healthy controls (Figure 4A). These results suggest that increased plasma levels of KL-6 in patients with ARDS reflect the pathophysiology of lung injury, rather than representing a nonspecific effect of mechanical ventilation or critical illness. Furthermore, these results are not explained by the incidence of other conditions known to cause elevated KL-6 levels in plasma (e.g. diabetes mellitus, malignant disease or other interstitial lung disease).
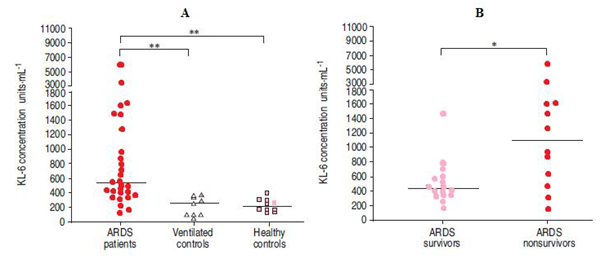
Figure 4 Plasma KL-6 concentrations in A) patients with acute respiratory distress syndrome (early sample), ventilated controls and healthy controls, and B) ARDS survivors and non-survivors (early samples).
Horizontal line indicates median value. *: pv0.05; **: pv0.001.9 It had been found that there is a significant difference in KL-6 levels between survivors and non-survivors of ARDS has been demonstrated (Figure 4B). Lung injury and primary respiratory failure are not considered to be principal causes of mortality in patients with ARDS, and the majority of non-surviving patients were died from multiple organ failure. It was also found that significant relationships have been shown between plasma KL-6 concentrations and certain ventilatory parameters. KL-6 levels correlated with peak and mean airway pressure, and there was a trend towards a statistically significant relationship with tidal volume, possibly indicating an association between ventilatory strategy and alveolar cell damage in ARDS. Anyhow, it is suggested that elevated levels of plasma KL-6 may provide a useful marker for acute respiratory distress syndrome in ventilated patients. Secondly, the finding that plasma KL-6 levels relate to severity of lung injury and mortality suggests a link between disruption of the alveolar epithelial lining and a poor outcome in acute respiratory distress syndrome. Finally, the relationship between plasma KL-6 concentration and airway pressures in mechanical ventilation is consistent with the hypothesis that ventilatory strategy influences alveolar epithelial damage in this syndrome.9
Pre-Elafin as a biomarker in ARDS
By using a microarray-based global gene profiling of whole blood total RNA, 126 genes was identified with altered expression between the acute stage and recovery stage in subjects with ARDS. Based on Gene Ontology annotations, the ARDS-related gene expression changes were clustered in gene ontology consortium [GO] biological processes related to response to stimulus (GO: 50896) and death (GO: 16265). Significant subordinate GO terms of death (GO: 16265) included cell death (GO: 8219), programmed cell death (GO: 12501), apoptosis (GO: 6915), regulation of programmed cell death (GO: 43067) and regulation of apoptosis (GO: 42981). This clustering of differentially expressed genes related to the regulation of apoptosis is supporting the observations of reduced apoptosis in alveolar neutrophils isolated from patients with ARDS. Anyhow, among the 126 identified genes, it was found that gene PI3 had the largest differential expression in peripheral blood of patients with ARDS. Expression of PI3 gene was suppressed at the early, acute stage of ARDS compared with significantly higher levels during the recovery stage of ARDS. PI3 gene encodes protein peptidase inhibitor 3 with two isoforms, including a 95–amino acid molecule named pre-Elafin (also known as trappin-2) and a 58–amino acid molecule called Elafin, which was produced by proteolytic cleavage of pre-Elafin. It was found that the PI3 protein expression is induced by inflammation-initiating cytokines and localized to the site of inflammatory response, including airways, skin, and other mucosal surfaces. However, it was found that the expression of PI3 gene in peripheral blood was differentially expressed between the acute stage and the recovery stage of ARDS.
Each ARDS case provides on one plasma sample. Based the date of sample collection relative to the ARDS diagnosis date, including pre-diagnosis group (Day 25 to Day 21), day of diagnosis group (Day 0), and post-diagnosis group (Day 1 to Day 3). Sample 1 of control was collected during the first 2 days of ICU admission. There was no statistically significant difference in baseline characteristics between patients with ARDS and control subjects, except that patients with ARDS more frequently received transfusion (P=0.009). Significant results of t test: ARDS pre-diagnosis versus post-diagnosis, P=0.0009.10
Importantly, the microarray findings of lower PI3 gene expression during the acute stage of ARDS was well correlated with the results of the ELISA assay measurements of plasma PI3 levels, which found PI3 was expressed at the lowest level in plasma during the acute stage, compared with plasma PI3 levels pre-diagnosis and the day of ARDS diagnosis. Moreover, the time course of plasma PI3 decrease was also well correlated with the course of early ARDS development, as the plasma sampling date relative to ARDS diagnosis not only shows a moderate negative correlation with plasma PI3 level, but also has the largest contribution in explaining the plasma PI3 variance in the multivariable linear regression model. Furthermore, there seems to be a pre-onset increase of the plasma PI3 level in the prediagnosis ARDS group of patients (mean 193.2pg/ml, 95% C: 124.4–262.2pg/ml; P 5 0.01), as compared with at-risk controls within the first 24-hours ICU admission (mean 97.6pg/ml, 95% C: 70.1–125.2pg/ml), even though it was not reached significantly level under multiple comparison adjustment (Figure 5). In contrast, there was a significant but moderate increase of plasma PI3 levels during a 3-days period after ICU admission in at-risk control subjects. Taken together, these observations suggest that PI3 levels in plasma could be used as a biomarker for monitoring the development and progress of ARDS. Nevertheless, neutrophils play a crucial role in the initiation and propagation of ARDS. Considerable evidence exists for the role of neutrophil-derived proteinases in the pathogenesis of ARDS, including neutrophil elastase and collagenase. A local imbalance between proteinases and their physiological inhibitors results in pulmonary parenchyma damage by leakage of a protein-rich fluid into the interstitium and alveolar spaces. Major pulmonary proteinase inhibitors include a1-proteinase inhibitor (a1-PI), secretory leukocyte proteinase inhibitor (SLPI), and elafin (PI3). Unlike high-molecular-weight a1-PI, which is mainly produced by the liver and reaches the lung via passive diffusion, SLPI and PI3 are low-molecular-weight inhibitors and are produced locally at neutrophil infiltration site in the lung. PI3 and SLPI are important anti-proteinases in the lung in both health and disease and also demonstrate multiple biological functions, such as antibacterial activity anti inflammatory activity, priming of innate immunity, tissue remodelling and cellular differentiation, and augmentation of antiviral adaptive immunity.10
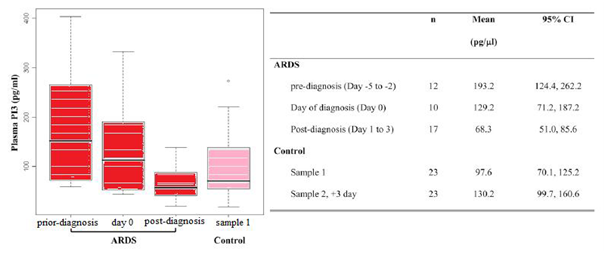
Figure 5 Levels of pre-Elafin (PI3) in plasma of patients with ARDS and critically ill patients who did not develop ARDS (control subjects).
Of 11 pairs of RNA samples used in microarray analysis, only 6 pairs had enough RNA for quantitative RT-PCR analysis, including 5 patients with acute respiratory distress syndrome (ARDS) and 1 control subject. Gene PI3, IL8 were chosen as having large differential expression between acute stage and stable stage of ARDS, as well as being identified repeatedly in Medline literature mining. The y-axis displays the fold change of the recovery stage to the acute stage of ARDS.10 For instance in animal studies it was found that in a murine model of lung injury mediated by Pseudomonas aeruginosa, intra tracheal administration of human elafin encoded on an adenovirus vector showed significant protection against lung injury by reduced protein concentrations in BAL fluid, increased elimination of bacteria from the airways, and decreased incidence of hematogenous bacterial dissemination.11 It was also found that a recombinant human pre-elafin exhibited a significant protective effect against human neutrophil elastase (HNE) induced acute lung injury in hamsters in a dose-dependent manner.
It was also found that gene IL8 had a large differential expression in peripheral blood of patients with ARDS, similar to the pattern of gene PI3. IL-8 is a potent neutrophil chemo attractant, which has been associated with ARDS in a number of previous studies. Markedly increased IL-8 level was consistently found in BAL fluid from patients with ARDS, with strong correlation to the numbers of BAL neutrophils, suggesting that IL-8 played a major role in promoting lung damage. Persistent increase of BAL IL-8 level was also associated with poor clinic prognosis. It was found that IL8 gene expression was suppressed at the early, acute stage of ARDS, as compared with the recovery stage (Figure 6).10
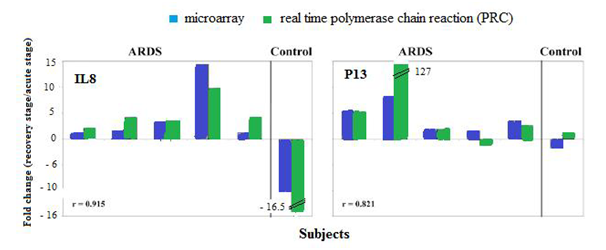
Figure 6 Comparison of the expression fold-changes of selected genes measured by microarray and quantitative RT-PCR.
Clara cell protein (CC16), a marker of lung epithelial injury. Clara cell secretory protein [which also known as CC16 and CC10] is a secreted product of the respiratory epithelium that within the lung is produced primarily from the Clara cells of the distal respiratory and terminal bronchioles. The biological function of CC16 remains incompletely understood, although CC16 has been demonstrated to interact with multiple components of the inflammatory and coagulation cascades. CC16 inhibits phosphor lipase A2 activity in vitro and in vivo, suggesting it plays a role in attenuating inflammatory responses. CC16 has also been implicated in feedback inhibition of interferon gamma signaling, as well as modulation of T helper 2 responses to pro inflammatory stimuli.
Furthermore, CC16 appears to be activated by tissue transglutaminases including activated Factor XIII, and inhibits thrombin-stimulated platelet aggregation, suggesting a possible role in modulating the dysregulated coagulation characteristic of ALI/ARDS. However, the CC16 has been investigated as a potential biomarker of lung epithelial injury in numerous disease states including idiopathic pulmonary fibrosis, sarcoidosis, COPD, asthma, occupational or environmental lung injury, bronchiolitis obliterans, chronic tobacco use and ALI/ARDS. Horizontal line represents median, box includes 25th to 75th percentile, error bars include tenth-ninetieth percentile.12
It was found that in patients with ALI/ARDS the levels of the anti-inflammatory CC16 were significantly reduced in both the pulmonary edema fluid and the plasma of patients with ALI/ARDS compared to a patients with cardiogenic pulmonary edema [CPE]. For instance in animal studies it was found that rats which exposed to intra tracheal lipopolysaccharide (LPS), a dose-dependent reduction in BAL fluid CC16 concentration was reported 24 hours after LPS administration. Nearly 10-folds reductions in lung homogenate CC16 were demonstrated, along with markedly decreased CC16 staining within terminal bronchioles by immunohistochemical analysis and reduced total lung homogenate CC16 messenger RNA. Reduced CC16 messenger RNA expression has also been reported in an acid aspiration rat model of ALI/ARDS. However, in observational studies of post-cardiopulmonary bypass patients at risk for ALI/ARDS, the mean CC16 levels in BAL fluid were not different between patients with ARDS and those at risk. Interestingly, although it was found that plasma CC16 concentration to be lower in patients with ALI/ARDS than those with cardiogenic pulmonary edema, the studies in animal as well as humans have shown acute increases in plasma CC16 concentration following inspired or intra tracheal LPS administration. Additionally, a study of critically ill mechanically ventilated patients found higher plasma CC16 levels in patients with ALI/ARDS compared to those at risk, and patients with ALI/ARDS who died had higher CC16 levels than survivors.13
Generally compared to healthy control subjects, patients with cardiogenic pulmonary edema do have increased total protein in BAL fluid, suggesting that some modest alteration in alveolar epithelial permeability occurs permitting bulk-flow transit of interstitial fluid into the airspace during the alveolar flooding phase of cardiogenic pulmonary edema without permitting transit of alveolar proteins into the interstitial and vascular spaces. It was found that the ratio of CC16 to total protein, comprised largely of albumin and other larger proteins in pulmonary edema fluid was significantly lower in patients with ALI/ARDS than those with cardiogenic pulmonary edema, suggesting that alternative mechanisms of alveolar flooding in ALI/ARDS and cardiogenic pulmonary edema may be responsible for different relative concentrations of CC16 and total edema fluid protein between the two disorders. However, lung injury is characterized by dynamic changes in the composition of the bronchial epithelium, including sloughing of Clara Cells in the acute phase and added differentiation and spreading of the ciliated epithelial cells, along with altered transcriptional activity within ciliated and non-ciliated epithelial cells. Furthermore, high levels of inspired oxygen commonly required and employed in patients with ALI/ARDS, decrease CC16 transcription through a hyperoxia-sensitive promoter element. Thus it was believed that the following three potential mechanisms explain why CC16 levels decrease in the setting of ALI/ARDS: the alterations in alveolar epithelial permeability, Clara cell death and changes in transcriptional activity within remaining Clara cells.12
Receptor for advanced glycation end-products [RAGE] a marker of acute lung injury
The receptor for advanced glycation end-products (RAGE) is one of the alveolar type I cell–associated proteins in the lung. Although it is also detected in endothelial cells in large vessels and nervous tissues, but the transcript of RAGE is most prominent in the lung and apparently not expressed in lung micro vascular endothelia. Immunoelectron microscopy of RAGE demonstrated that its expression is localized to the basal membrane of alveolar type I epithelial cells. This protein is belongs to the immunoglobulin super family of cell-surface molecules, consisting of an extracellular region (one V-type ligand binding site and two C type immunogloblin-like regions), a trans membrane domain, and a cytosolic tail, that are essential for post-RAGE signaling. In general, RAGE is a multi-ligand-binding receptor that an bind advanced glycation end products, amyloid b-peptide, S100 proteins, and high mobility group box-1. RAGE–ligand interaction results in intracellular signaling, which leads to activation of the pro inflammatory transcription factor nuclear factor-kB (NF-kB). This cellular activation is related to inflammatory processes or tissue injury, such as diabetic micro vascular injury, amyloidosis, and immune-inflammatory process. Alveolar type I epithelial cells cover more than 95% of the internal surface area of the lung, and damage to alveolar type I epithelial cells is an important feature of acute lung injury and acute respiratory distress syndrome. In this context, some alveolar type I epithelial cell proteins have been demonstrated to be markers of the severity of lung injury in animal and human studies. Because of the new evidence regarding high concentrations of RAGE in the lung apparently localized primarily to alveolar epithelial type I cells, it was hypothesized that RAGE may be a useful biochemical marker of alveolar type I epithelial cell injury in ALI and ARDS.
RAGE levels in the pulmonary edema fluid from patients with ALI/ARDS were significantly higher than levels in the patients with severe hydrostatic pulmonary edema. Plasma RAGE levels in patients with ALI/ARDS were also significantly higher than the level in the healthy volunteers and in the patients with severe hydrostatic pulmonary edema. These findings indicate that RAGE may be useful as a marker of alveolar epithelial type I cell injury in the acutely injured lung.
Since its first purification from a bovine lung c DNA library, RAGE has been found to be most abundant in the lung. As several recent studies demonstrated, RAGE seems to be expressed in the lung exclusively in alveolar type I epithelial cells and its expression is localized to the basolateral membrane in the normal lung. It was demonstrated that neither alveolar type II epithelial cells nor alveolar macrophages expressed RAGE under lipopolysaccharide-stimulated conditions or in the normal lung. Another possible source of RAGE is the lung endothelium. However, human lung micro vascular endothelial cells in culture demonstrated no expression of RAGE regardless of lipopolysaccharide stimulation.
Although there are extra pulmonary sources of RAGE, the vast majority of RAGE seems to be from the lung because the RAGE levels in the BAL fluid in the pulmonary edema fluid in the patients were much higher than levels in the circulation. In the setting of less severe lung inflammation, RAGE was not detectable in the serum despite its presence in the BAL fluid. These results strongly suggest that the source of RAGE in the alveolar space and in the serum was primarily alveolar type I epithelial cells. It was reported that bleomycin-induced lung injury caused significant loss of the expression of RAGE in the lung tissue after 7 days of bleomycin administration. These experimental data support the hypothesis that the major source of RAGE in the lung is the alveolar type I epithelial cell because the number of intact alveolar type I epithelial cells is significantly decreased after bleomycin administration and because expression of other alveolar type I epithelial cell specific proteins, such as aquaporins 5 and RTI40, were also decreased.14
Because the source of RAGE is the alveolar type I epithelial cell, it seems likely that the significant increase in the RAGE levels in the edema fluid in ALI/ARDS compared with hydrostatic pulmonary edema reflected enhanced release from the alveolar type I epithelium. Furthermore, RAGE levels in the plasma from patients with ALI/ARDS were significantly higher than in the plasma of healthy volunteers and patients with hydrostatic pulmonary edema. Although the plasma RAGE levels were 100 times lower in the plasma than in the edema fluid, plasma RAGE levels might also reflect the injury to alveolar epithelial type I cells. Although the levels were lower than those in patients with ALI/ARDS, the detection of RAGE in the edema fluid and the moderate elevation of RAGE in the plasma of patients with severe hydrostatic pulmonary edema suggests that there may be some low-level injury to the alveolar epithelium in these patients that may be related to the stress-induced epithelial changes reported or to the effects of positive pressure ventilation in patients with severe lung edema.
However, because of its important role in the barrier function between airspace and lung tissue, structural abnormalities of alveolar type I epithelial cells are an important factor in the pathogenesis of ALI/ARDS. For example, recent studies suggested a possible role for type I alveolar epithelial cells in alveolar fluid clearance and structural properties of alveolar type I epithelial cells are also critical for maintaining the normal barrier properties of the alveolar capillary barrier. Therefore, biochemical markers that reflect abnormalities of alveolar type I epithelial cells have the potential to improve the detection and evaluation of severity of ALI/ARDS.15
Elevated cardiac troponin-T levels in acute respiratory distress syndrome patients
The Acute Respiratory Distress Syndrome is marked by considerable cardiovascular strain. This is related to severe respiratory compromise and hypoxemia caused by endothelial injury and capillary leak, which result in alveolar filling and subsequent respiratory failure. Hypoxemia stimulates increased cardiac output, which can result in myocardial strain in the setting of decreased oxygen supply and pulmonary hypertension due to hypoxic pulmonary vasoconstriction, pulmonary endothelial injury, and in situ thrombosis of the pulmonary vasculature. This condition is further exacerbated by pulmonary capillary obliteration and lung fibrosis as the syndrome progresses, as well as by increased intra-thoracic pressure from mechanical ventilation. Patients with sepsis and septic shock, which are commonly associated with ARDS, are known to have a syndrome of myocardial dysfunction at least partly due to inflammation-induced myocardial apoptosis. As well as myocardial dysfunction may be exacerbated by increased myocardial oxygen demand during critical illness, in the condition of pre existing coronary heart disease which is restricting coronary arterial blood flow. In general while ARDS is considered a pulmonary disease, the cardiovascular consequences from this devastating illness are substantial. Echocardiography is frequently used in the setting of critical illness to assess cardiovascular structure and function. Patients with sepsis and septic shock undergo echocardiography to evaluate the etiology of pulmonary edema and ventricular dysfunction, and the sensitivity of trans-thoracic echocardiography for cardiac causes of shock has been reported to be as high as 100%. It was well known that the changes over time in pulmonary artery hemodynamics have been associated with prognosis in critically ill patients with acute severe respiratory failure. Likewise, ECG is an important and ubiquitous diagnostic tool in the intensive care unit and has been shown to perform reasonably well in detecting myocardial ischemia and infarction in ICU patients. Though it has been demonstrated that elevated levels of cardiac biomarkers are associated with increased mortality and length of stay in ICU populations, the underlying cause of such biomarker elevations remains to be controversy.16 Troponin assays are widely used for their diagnostic and prognostic utility in several clinical settings, including detection or exclusion of myocardial infarction, heart failure and pulmonary embolism, and as supportive tests in sepsis and stroke. It has previously shown that elevated levels of blood biochemical markers of myocardial necrosis are associated with increased morbidity and mortality in ARDS patients.
It had been shown that cardiac troponin T [cTnT] levels are frequently elevated in patients with ARDS, and that cTnT elevation is associated with adverse outcomes, including death, organ failure, and need for mechanical ventilation (Figure 8). The association of increasing cTnT level with mortality from ARDS remains consistent after adjustment for other factors involved in outcome and is valid through a variety of statistical analyses. In addition, it has shown that there were few significant differences in ECG and echo cardio graphic parameters between patients with ARDS with and without elevated cTnT. In particular, it did not appear that patients with ARDS and elevated cTnT are having missed classical (or type 1) acute myocardial infarctions or other variants of the acute coronary syndrome. There are multiple mechanisms whereby myocardial necrosis may occur in ARDS patients. It is logical that the pathogenesis is near to that of myocardial injury sustained by patients with severe sepsis/septic shock. However, these results show that many ARDS patients had detectable cTnT levels even in the absence of suspected infection. Moreover, plasma cTnT had independent value in predicting mortality when compared with the myriad clinical data represented by the APACHE III score. This suggests that myocardial necrosis could be important in the pathogenesis of ARDS beyond being a hallmark of systemic disease such as septic shock. However, some of the molecular mechanisms postulated to be involved in septic myocardial dysfunction may also be in play in ARDS even in the absence of infection or shock, including circulating pro inflammatory mediators, nitric oxide activity, matrix metalloproteinase activation, mitogen-activated protein kinase activity, and induction of cellular apoptosis.
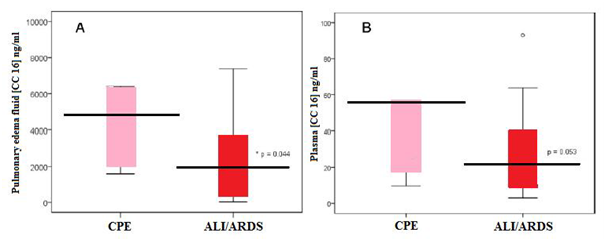
Figure 7 Box plot summary of CC16 levels in pulmonary edema fluid (A) and plasma (B) of patients with CPE or ALI/ARDS.
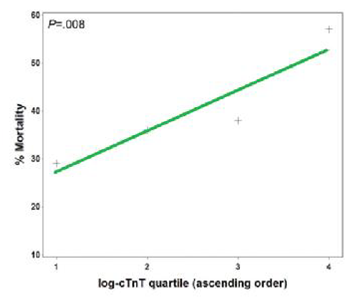
Figure 8 Relationship between cTnT log quartile and mortality, with super imposed trend line. (P value stated for Cochran-Armitage test of trend).
However, another possible explanation for these findings is that flow-limiting atherosclerotic lesions due to coronary heart disease may predispose individuals to ischemic myocardial necrosis in the setting of increasing myocardial oxygen demand and reduced coronary blood flow due to critical illness (so-called type II myocardial infarction). Supporting this explanation are the findings of more regional wall motion abnormalities and a trend toward more pathologic Q waves in the patients with elevated cTnT, which may indicate a greater penetrance of coronary heart disease and flow-limiting coronary lesions in that patients. Contradicting this explanation, however, are data that coronary blood flow is increased, not decreased, in patients with septic shock, and that elevated troponin levels in a majority of critically ill patients were not associated with flow-limiting atherosclerotic coronary artery lesions in a prior study. Furthermore, we did not observe significant differences between patients with and without cTnT elevations with respect to any ECG signs of active myocardial ischemia or acute coronary syndromes. While patients with elevated cTnT were more likely to have regional wall motion abnormalities on echocardiography, which can be present in both active cardiac ischemia and in patients with a history of myocardial infarction, this finding is not specific to coronary heart disease and was seen in a minority of patients. It is possible that patients with ARDS may be at risk of demand-related myocardial ischemia independent of coronary heart disease. In this scenario, cardiac biomarker elevation from such type II myocardial infarctions may not be associated with classic ECG signs of ischemia or specific findings on echocardiography.17 Pulmonary vascular disease and associated right ventricular strain are likely contributors to myocardial necrosis in ARDS. In particular, pulmonary vascular disease is known to accompany ARDS, and hemodynamic evidence of right ventricular strain has been implicated in poorer prognosis. It had been shown that increased pulmonary dead space and radiologically-determined pulmonary vascular obstruction are associated with worse outcomes in ARDS. It also had shown that patients with elevated cardiac biomarkers are no more likely to show right ventricular hypokinesis, right ventricular dilation, or elevated right ventricular systolic pressure on trans-thoracic echocardiography, than are patients with normal levels of cTnT. It was found that between22 and 33% of patients had evidence of right ventricular dilation or hypokinesis, which is very similar to previously reported rates of echo cardio graphically demonstrated acute cor pulmonale in patients with ARDS. There are other potential explanations for the lack of correlation between cTnT and right ventricular dysfunction:
Exhaled breath condensate biomarkers in acute lung injury
Collection of exhaled breath condensate (EBC) is a novel non invasive means of obtaining lower respiratory tract samples that can be repeated several times with short intervals between sampling. The collection devices can be used in patients breathing spontaneously as well as in mechanically ventilated patients. The technique is based on the hypothesis that particles exhaled in breath reflect the composition of the alveolar lining fluid. Therefore, the inflammatory markers and several molecules can be detected in exhaled breath condensate. The concentration of these mediators is influenced by lung diseases and may be modulated by therapeutic interventions; hence, exhaled breath condensate analysis could be a useful, non invasive technique for monitoring the evolution of lung diseases. Really a several studies have reported mediator changes in exhaled breath condensate samples from acute respiratory distress syndrome patients, such as an increased hydrogen peroxide concentration and an increased 8-isoprostane (8-isoPGF2α) concentration in patients with, or at risk for, ARDS as compared with normal control subjects. A correlation between the exhaled breath condensate nitrite concentration and the tidal volume has been found in acute lung injury patients, possibly reflecting ventilator-associated lung injury. The exhaled breath condensate pH has been related to the extent of lung injury, and a good correlation with exhaled breath condensate IL-6 and IL-8 concentrations has been observed. In addition, ALI and ARDS patients show higher EBC cytokine concentrations than healthy volunteers. It was found that the potential benefits of β-adrenergic stimulation in ALI include epithelial protection, decreased neutrophil chemotaxis and activation, lower pro inflammatory cytokine production, increased surfactant secretion, improved respiratory mechanics, and increased alveolar fluid clearance.
The pH of the airway lining fluid is the result of a balance between different buffer systems and the production and release of acids and bases in the airways. In a healthy airway, several factors favour acidification of the airway lining fluid, such as secretion by alveolar type 2 cells and macrophages, necrosis of macrophages and the alveolar carbon dioxide partial pressure (pCO2). The pH becomes more alkaline in the proximal airway owing to airway epithelial cell enzyme systems, ion channel activity and buffering proteins. It was confirmed that the normal range of exhaled breath condensate pH for healthy subjects is 7.4 to 8.8. Few studies have reported exhaled breath condensate pH values for mechanically ventilated patients. In otherwise healthy patients undergoing lung resection for cancer, the mean exhaled breath condensate pH obtained using RTubes™ was 7.8. Another studies of patients undergoing cardiothoracic surgery that used the same exhaled breath condensate collection device reported pH values between 5 and 7. In general, using the Eco Screen in mechanically ventilated patients, a mean de-aerated exhaled breath condensate pH value of 5.98 was reported. Anyhow, three essential mechanisms in ALI patients may lead to exhaled breath.
Condensate Acidification:
Exhaled breath condensate biomarkers before and after salbutamol administration.18It was found that the exhaled breath condensate acidity is an important marker of lung inflammation and the higher exhaled breath condensate pH values detected after a single dose of salbutamol could be related to an anti-inflammatory effect of β-adrenergic stimulation in ALI patients (Table 2). However, no significant changes in the partial pressure of arterial carbon dioxide after salbutamol inhalation were observed, which means that changes observed in the pH were not caused by changes in alveolar ventilation. In recent studies, exhaled breath condensate pH values were continuously monitored in mechanically ventilated patients, and exhaled breath condensate acidification was observed even before the clinical alteration appeared. This phenomenon has also been observed in studies on chronic obstructive pulmonary disease and asthma xacerbations, bronchiestasis, cystic fibrosis and other studies on ALI.
Salbutamol |
P value |
||
Before administration |
After administration |
||
7.66 |
7.83 |
0.028 |
|
Conductivity (us) |
74 |
64 |
0.465 |
Leukotrienes B4 (pg/ml) |
1.58 |
2.06 |
0.753 |
Nitrite (uM) + nitrate (uM) |
18.81 |
21.21 |
0.173 |
8-isoprostane (pg/ml) |
11.64 |
6.55 |
0.068 |
Table 2 Exhaled breath condensate biomarkers before and after salbutamol administration
A trend towards decreased nitrosative species and 8-isoPGF2α was observed after a single dose of salbutamol. Several inflammatory mediators are present in exhaled breath condensate samples, such as interleukins, leukotrienes, reactive oxygen and nitrogen species. Pulmonary nitric oxide production has been shown to be stimulated by mechanical forces. Release of pulmonary nitric oxide species may reflect alveolar distension and inflammation. In fact, exhaled breath condensate nitrite has been closely correlated to tidal volume, and the exhaled breath condensate nitrite and tidal volume ratio has been strongly correlated to the extent of lung injury, using the oxygenation criteria of the consensus definition or the Lung Injury Score. The 8-isoPGF2α is a marker of oxidative stress in patients with asthma, interstitial lung disease, chronic obstructive pulmonary disease, cystic fibrosis and ALI. However it was suggested that salbutamol inhalation may play a role in preventing the lipid per-oxidation that occurs in ALI and ARDS patients.18
Markers of lung injury before and after positive pressure ventilation
Despite advances in intensive care, ALI/ARDS is associated with a high mortality rate of 35% - 40% and an incidence of approximately 200,000 cases per year. However, the only main therapeutic modality that has improved the survival in ALI/ARDS is a lung-protective ventilation strategy. Nevertheless, the mechanisms by which a lung-protective ventilation strategy confers a mortality benefit are relatively incompletely understood, but a reduction of the lung injury that leads to the release of pro-inflammatory cytokines is one likely mechanism. Structural disruption of the lung caused by mechanical ventilation (barotraumas and volutrauma) includes a component of associated mediator release (bio trauma) which can further aggravate lung injury and potentially lead to systemic multi-organ failure. Plasma levels of interleukin (IL)-6, IL-8, surfactant protein D (SP-D), and soluble tumor necrosis factor receptor I/II (sTNFrI/II) are elevated in patients with ALI/ARDS, their levels change in response to different ventilation strategies, and interestingly, this response is rapid. Furthermore, baseline levels of IL-6, IL-8, SP-D, intercellular adhesion molecule-1 (ICAM- 1), von Willebrand factor (vWF), and TNFrI/II in patients with ALI/ARDS are associated with worse clinical outcomes.
However, in patients with ALI/ARDS who are spontaneously ventilating with supplemental oxygen, it is not known whether the institution of positive pressure ventilation exacerbates the pre existing lung injury. It is possible that endotracheal intubation followed by the institution of a lung-protective ventilation strategy with a lower tidal volume and a plateau pressure less than 30cm H2O would not worsen already established ALI/ARDS. On the other hand, it is also possible that the institution of even a lung-protective positive pressure ventilation strategy would worsen lung injury simply because the injured alveoli are exposed to some level of positive airway pressure. It was reported that plasma cytokine levels in patients with ALI/ARDS change within 1 hour of a change in ventilation strategy.
It was found that generally, the serum levels of IL-8, IL-6, vWF, and ICAM-1 were significantly elevated in spontaneously ventilating patients with ALI/ARDS prior to the institution of positive pressure ventilation. Furthermore, it was indicated that the institution of a lung-protective ventilation strategy in patients with ALI/ARDS did not significantly increase the serum levels of IL-8, IL-6, vWF, and ICAM-1 (Figure 9A–D).
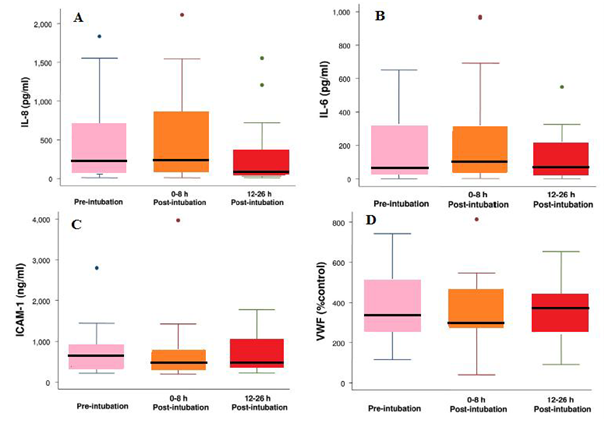
Figure 9(A) Box plot summary of interleukin (I L)-8 levels. Median levels of IL-8 were 235pg/ml (range, 10 to1, 836pg/ml) pre-intubation, 219 pg/ml (range, 10 to 2,115pg/ml) immediately post-intubation and 68pg/ml (range, 10 to 1,552pg/ml) at 12 to 26 hours post-intubation. The decrease in IL-8 level at 12 to 26 hours after intubation was statistically significant (p=0.002, paired t test with Bonferroni correction for multiple comparisons). The horizontal line represents the median, the box encompasses the 25th to 75th percentile, and error bars encompass the 10th to 90th percentile.
(B) Box plot summary of interleukin (I Ll)-6 levels. Median levels of IL-6 were 76pg/ml (range, 3 to 652pg/ml) pre-intubation, 132pg/ml (range, 4 to 971pg/ml) immediately post-intubation and 90pg/ml (range, 3 to 550pg/ml) at 12 to 26 hours post intubation. There was no difference among the levels of IL-6 at the three different time points (p=0.34).
(C) Box plot summary of intercellular adhesion molecule-1 (ICAM-1) levels. Median levels of ICAM-1 were 631ng/ml (range, 220 to 2,800ng/ml) pre-intubation, 520ng/ml (range, 198 to 3,970ng/ml) immediately post-intubation and 492ng/ml (range, 221 to 1,780ng/ml) at 12 to 26 hours post-intubation. There was no statistically significant difference among the levels of ICAM-1 at the three different time points (p=0.15).
(D) Box plot summary of von Willebralnd factor (vWF) level is expressed as a percentage of a normal pooled plasma control reference. Median levels of vWF were 368% (range, 116% to 742%) pre-intubation, 312% (range, 40% to 814%) immediately post-intubation and 359% (range, 91% to 653%) at 12 to 26 hours post intubation. There was no statistically significant difference among the levels of vWF at the three different time points (p=0.57).
However, ALI/ARDS is characterized by injury to the lung endothelial and alveolar epithelial barriers, pulmonary edema, release of inflammatory mediators, and non-pulmonary organ failure. Several biomarkers of inflammation (IL-6, IL-8, and sTNFrI/II) and epithelial (SP-D) and endothelial (vWF) injury as well as adhesion molecules (ICAM-1) have been shown to be predictors of morbidity and mortality in patients with ALI, indicating that the levels of these biomarkers are affected by the severity of the lung injury. Positive pressure mechanical ventilation imposes cyclic pressure and volume stress on the lung which can disrupt the pulmonary architecture and lead to the release of inflammatory cytokines. It was found that the high tidal volumes can precipitate lung injury and can be associated with increased cytokine production and extra-pulmonary organ damage. Anyhow, in healthy human, short-term mechanical ventilation has not been shown to be associated with cytokine release, regardless of the ventilation strategy. However, in ventilated patients with established lung injury, the ventilation strategy has been shown to impact cytokine levels. It was found that patients with ARDS who managed with a conventional (11.1 ml/kg, positive end-expiratory pressure 6.5) and lung-protective (7.6ml/kg, PEEP 14.8) ventilation strategies and measured bronchoalveolar lavage and plasma biomarker levels at baseline and at 36 hours after intubation. BAL fluid and plasma levels of sTNFrI, sTNFrII, IL-6, and tumor necrosis factor-α (TNF-α) at 36 hours were significantly lower in the low tidal volume patients compared with the high tidal volume group of patients. Based on this observation, it is concluded that mechanical ventilation itself can lead to an increase in cytokine levels in the lung as well as systemic circulation. Interestingly, it was demonstrated that in patients with lung injury that a higher tidal volume ventilation strategy (12ml/kg, PEEP of 5cm H2O) for only six hours was associated with a significant increase in plasma IL-6, IL-10, TNF-α, and IL-1ra compared with the initial low tidal volume strategy (6ml/kg, PEEP of 15cm H2O) and also that restoration of the low tidal volume strategy resulted in a decrease of the biomarker levels back to baseline.19
None.
The authors declare there are no conflicts of interest.
None.

©2016 Zwer. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.