Journal of
eISSN: 2373-6437


Review Article Volume 5 Issue 5
Classified Specialist/Associate Prof Anesthesiology & Intensive Care, Combined Military Hospital, Bangladesh
Correspondence: Lt Colonel Abul Kalam Azad, Classified Specialist/Associate Prof Anesthesiology & Intensive Care, Combined Military Hospital, Post Code-1206, Dhaka Cantonment, Dhaka, Bangladesh, Tel 008801715010956
Received: July 11, 2016 | Published: August 30, 2016
Citation: Kalam LCAA (2016) Simple Algorithm of Arterial Blood Gas Analysis to Ensure Consistent, Correct and Quick Responses!. J Anesth Crit CareOpen Access 5(5): 00199. DOI: 10.15406/jaccoa.2016.05.00199
Background: Arterial blood gas (ABG) analysis is an essential part of diagnosing and managing a patient's oxygenation, ventilation status as well as acid-base balance. The usefulness of this diagnostic tool is dependent on being able to correctly interpret the results. The body operates efficiently within a fairly narrow range of blood PH (acid-base balance). Even relatively small changes can be detrimental to cellular function. Disorders of acid-base balance can create complications in many disease states, and occasionally the abnormality may be so severe so as to become a life-threatening risk factor. A thorough understanding of acid-base balance is mandatory for physicians, intensivists, and anesthesiologists are not exception! We must always interpret them in light of the patient’s history, clinical presentation and laboratory information’s.
Objectives: ABG is not merely a tracing paper! So many variables right at the tracing paper as well as clinical variables of the patients hatch fearfulness among young physicians. So the effort was to make ABG EASY and to develop an algorithm which will conduct navigating diagnosis!
Conclusion: Arterial blood gases help assess three vital physiologic processes in the critically ill patient: acid-base balance, ventilation and oxygenation. Initial blood gas analysis helps diagnose underlying disease processes as well as guide therapeutic interventions. Serial measurements can be utilized to assess proper response to therapy. Blood gas analysis takes a step-by-step approach and practice. Blood gas data should always be integrated in light of the full clinical and laboratory information.
Keywords: oxygenation, ventilation, acid-base, metabolic, saturation, bicarbonate
Arterial blood gas (ABG) analysis is a crucial part of diagnosing and managing a patient's state of oxygenation, ventilation as well as acid-base balance. The practicability of this diagnostic tool is dependent on being able to correctly interpret the results. Disorders of acid-base balance can create complications in many disease processes, and occasionally underlying disorders may be so severe that might cause life-threatening risk. So, thorough understanding of acid-base balance is vital for any physician, intensivist, and anesthesiologists are not exception.
ABG analysis is a diagnostic tool that allows the objective evaluation of a patient’s oxygenation, ventilation and acid-base balance. The results from an ABG will indicate not only patient’s respiratory status but also indicate how well a patient’s kidneys and other internal organs (metabolic system) are functioning. Although all of the data in an ABG analysis can be useful, it is possible to interpret the results without all variables. Essentials of interpreting ABG need maximum of six values: - Oxygen concentration (PO2), - Oxygen saturation (SaO2), - Bicarbonate ion concentration (HCO3-), - Base excess, - Carbon dioxide concentration (PCO2); - Hydrogen ion concentration (PH).1–5
Basic terminology6–8
Requirement of acid-base balance7,8
Acid-base balance is important for metabolic activity of the body:
PH of arterial blood = 7.35 – 7.45.
Alteration of PH value out of the range 7.35-7.45 will have effects on normal cell function.
PH< 6.8 or > 8.0 death occurs.

Changes in excitability of nerve and muscle cells
↓PH→ depresses the CNS
Can lead to loss of consciousness.
↑PH → over-excitability of CNS
Tingling sensations, nervousness, muscle twitches.

Alteration of enzymatic activity:
PH change out of normal range can alter the shape of the enzyme rendering it non-functional.

Alteration of K+ levels
Acid-base state of ECF influence:
K+ distribution in ECF and ICF
Renal excretion of K+
Acid-base disturbance or imbalance

Acid-base disturbances:


Regulation of acid-base balance
React very rapidly (less than a second)
Reacts rapidly (seconds to minutes)
Reacts slowly (2~4 hours)
Reacts very slowly (12~24 hours)
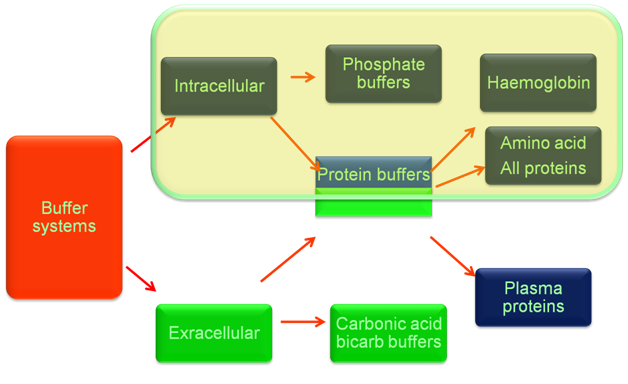
Respiratory regulation
The lung regulates the ratio of [HCO3-]/[H2CO3] to approach 20/1 by controlling the alveolar ventilation and further elimination of CO2, so as to maintain constant PH value.
Regulation of alveolar ventilation (VA)
Central chemoreceptor (located at medulla oblongata)
↑[H+] in Cerebrospinal fluid→ respiratory center exciting→ ↑VA
PaCO2> 60mmHg→ VA increase 10 times
PaCO2> 80mmHg→ respiratory center inhibited
Peripheral chemoreceptor (carotid and aortic body)
↓PaO2< 60mmHg→ respiratory center exciting→ ↑VA
↓PaO2< 30mmHg→ respiratory center inhibited
How does alteration of alveolar ventilation regulate PH value?
↑[H+] in Blood→ rapidly buffered by buffer system such as HCO3-/H2CO3→ ↓ [HCO3-] and ↑ [H2CO3] → [HCO3-]/[H2CO3] tend to decrease, while ↑[H+] can stimulate peripheral chemoreceptor →respiratory center exciting →↑alveolar ventilation →↑CO2 elimination →↓PaCO2 → [HCO3-] / [H2CO3] tends to 20/1 → PH is maintained.
Renal regulation
The kidney regulates [HCO3-] through changing acid excretion and bicarbonate conservation, so that the ratio of [HCO3-]/[H2CO3] approach 20/1 and PH value is constant.
Bicarbonate conservation
How does the renal regulation maintain the constant PH value?
↑[H+] in Blood→ rapidly buffered by buffer system such as HCO3-/H2CO3→ ↓ [HCO3-] and ↑ [H2CO3] → [HCO3-]/[H2CO3] tend to decrease, while ↑[H+] can stimulate the activity of CA, H+-ATPase and glutaminase→↑secretion of H+ and ammonia, ↑reabsorption of HCO3- → [HCO3-] / [H2CO3] tends to 20/1 → PH is maintained.
Ion exchange between intra- and extracellular compartment & intracellular buffering:
i.e. ↑Extracellular [H+] → H+ shift into cells and K+ shift out of cells
Base excess & base deficit9,10
In human physiology base excess and base deficit refer to an excess or deficit, respectively, in the amount of base present in the blood. The value is usually reported as a concentration in units of mEq/L, with positive numbers indicating an excess of base and negative a deficit. A typical reference range for base excess is −2 to +2mEq/L. Comparison of the base excess with the reference range assists in determining whether an acid/base disturbance is caused by a respiratory, metabolic, or mixed metabolic/respiratory problem.
The base excess of blood does not truly indicate the base excess of the total extracellular fluid (ECF). Because of different protein content and the absence of hemoglobin, ECF has a different buffering capacity. What’s more, each extracellular fluid (for example CSF vs interstitial fluid) has a different buffer status. The clinical determination of the amount of bicarbonate required for treatment of severe acidosis is usually based on the base excess of the blood.
There is an unavoidable inaccuracy, however, due to several factors:
In general, however, recommendations for bicarbonate therapy are in the range of 0.1 to 0.2 mEq times the body weight times the base excess (ignoring the minus sign).
Bicarb = 0.1 x (-B.E.) x Wt in Kg
Tips for determining primary and mixed acid base disorder11,12
Tip-1: Only a process of acidosis can make the PH acidic and only a process of alkalosis can make PH alkaline
Tip-2: In primary disorder PH 7.35 ─ 7.40 is indicative of primary acidosis, when compensation is complete
Tip-3: In primary disorder PH 7.40 ─ 7.45 is indicative of primary alkalosis, when compensation is complete
Tip-4: Keeps in mind that three states of compensation are possible:
Tip-5: Don’t interpret any blood gas data without examining corresponding serum electrolytes.
Tip-6: Truly normal PH with distinctly abnormal HCO3- and PaCO2 invariably suggests two or more disorders.
Tip-7: Whenever the PCO2 and [HCO3] are abnormal in opposite directions, ie, one above normal while the other is reduced, a mixed respiratory and metabolic acid-base disorder exists.
Facts about Acid-Base balance……13

Compensation of primary & mixed disorder
Compensation for simple acid-base disturbances always drives the compensating parameter (ie, the PCO2, or [HCO3-]) in the same direction as the primary abnormal parameter (ie, the [HCO3-] or PCO2) & compensation for mixed disorder always drives compensating parameters in the opposite direction as the primary abnormal parameters.

Description of superscripts inside algorithm box15–29
Delta ratio is a formula that can be used to assess elevated anion gap metabolic acidosis and to evaluate whether mixed acid base disorder is present.
In High anion gap metabolic acidosis (HAGMA) Delta ratio will be 1-2
If the ratio is greater than 2 in a HAGMA it is due to concurrent metabolic alkalosis.
In Nonanion gap metabolic acidosis (NAGMA) delta ratio will be =0.4
If the ratio is between 0.4-1 then it is due to Mixed (HAGMA+NAGMA) disorser
Bedside Rules for Assessment of Compensation14
Rule 1: The 1 for 10 Rule for Acute Respiratory Acidosis
The [HCO3] will increase by 1 mmol/l for every 10 mmHg elevation in pCO2 above 40 mmHg.
Expected [HCO3] = 24 + {(Actual pCO2 - 40) / 10}
Rule 2: The 4 for 10 Rule for Chronic Respiratory Acidosis
The [HCO3] will increase by 4 mmol/l for every 10 mmHg elevation in pCO2 above 40mmHg.
Expected [HCO3] = 24 + 4 {(Actual pCO2 - 40) / 10}
Rule 3: The 2 for 10 Rule for Acute Respiratory Alkalosis
The [HCO3] will decrease by 2 mmol/l for every 10 mmHg decrease in pCO2 below 40 mmHg.
Expected [HCO3] = 24 - 2 {(40 - Actual pCO2) / 10}
Rule 4: The 5 for 10 Rule for a Chronic Respiratory Alkalosis
The [HCO3] will decrease by 5 mmol/l for every 10 mmHg decrease in pCO2 below 40 mmHg.
Expected [HCO3] = 24 - 5 {(40 - Actual pCO2) / 10} ( range: +/- 2)
Rule 5: The One & a Half plus 8 Rule - for a Metabolic Acidosis
The expected pCO2 (in mmHg) is calculated from the following formula:
Expected pCO2 = 1.5 x [HCO3] + 8 (range: +/- 2)
Rule 6: The Point Seven plus Twenty Rule - for a Metabolic Alkalosis
The expected pCO2 (in mmHg) is calculated from the following formula:
Expected pCO2 = 0.7 [HCO3] + 20 (range: +/- 5)
Predicting difficult tracheal intubation is still a matter of debate among anesthesiologists, with each individual score or sign having a poor predictive value.1 In the present case report we present a case of difficult tracheal intubation that could not have been predicted with any score or clinical sign used for preoperative airway assessment.
None.
The authors declare there is no conflict of interests.

©2016 Kalam. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.