Journal of
eISSN: 2373-6437


Research Article Volume 4 Issue 3
1Anaesthesia Senior Resident, India
2Anaestheia Assitant Professor, India
3Anaesthesia Associate Professor, India
4Anaesthesia Senior Professor, India
Correspondence: Shveta Kajal, Department of Anaesthesiology and Pain clinic, Pt. B.D. Sharma PGIMS,599/30, Adarsh nagar, Rohtak, Haryana, India 124001, Tel +918750821207
Received: November 24, 2015 | Published: February 5, 2016
Citation: Kajal S, Dhankhar M, Mukherjee S, Arya G, Kumar P, et al. (2016) Evaluation of Low Doses of Neostigmine for Reversal of Residual Neuromuscular Blockade. J Anesth Crit Care Open Access 4(3): 00137. DOI: 10.15406/jaccoa.2016.04.00137
Neuromuscular blocking agents (NMB) are now used routinely to facilitate tracheal intubation, mechanical ventilation and for skeletal muscle relaxation during the entire surgical procedure. At the end of the surgical procedure, anticholinesterases along with anticholinergics are administered to reverse the neuromuscular blockade. Neostigmine causes almost complete antagonism of neuromuscular blockade and is the most potent agent. Routinely prescribed dose of neostigmine is 40-60µg kg-1, but the dose varies widely. Administration of neostigmine however is associated with systemic side effects e.g. bradycardia, nodal and ventricular escape beats, nausea, vomiting, increased salivation, increased bowel motility and bronchoconstriction etc. Potentiation of, rather than recovery from, neuromuscular block has been demonstrated when 40 µg kg-1 of neostigmine is administered after recovery to a TOF ratio ≥ 0.9 after administration of 0.1 mg kg-1 of vecuronium. Inadequate recovery resulting from low degree of residual paralysis is frequent, difficult to detect and potentially harmful. The present study on 120 healthy ASA I and II patients has been carried out to find out the most appropriate dose of neostigmine for adequate reversal of neuromuscular blockade. Recovery in patients receiving 40µg kg-1 was significantly faster but had no added advantage. There is a risk of drop in TOFR after adequate recovery in patients receiving high doses of neostigmine. It is found that group receiving 20µg kg-1 & 30µg kg-1 of neostigmine had reasonably fast and sustained recovery without significant adverse effect. Use of neostigmine in doses of 20µg kg-1- 30µg kg-1 seems to be adequate for complete recovery from neuromuscular blockade when given at TOF 0.4 without any adverse effects.
Neuromuscular blocking agents (NMB) are now routinely used to facilitate tracheal intubation, mechanical ventilation and skeletal muscle relaxation throughout the entire surgical procedures. Murphy et al reported that residual paralysis resulting from inadequate reversal is an important contributing factor to critical postoperative respiratory events (inadequate pulmonary functions, reduced pharyngeal muscle coordination, obstruction of upper airways, increased risk of aspiration and impaired hypoxic ventilatory response).1 Neostigmine (anticholinesterases) by increasing acetylcholine concentration at synaptic cleft causes more complete antagonism of neuromuscular blockade. Though the dose of neostigmine varies widely, 40-60µg kg-1 is routinely prescribed.2
Adequate reversal can be measured clinically (ability to open eyes widely, cough effectively, sustain tongue protrusion, hand grip, head lift and leg raising) when the patient is conscious and cooperative3 butideally, the response should be evaluated mechanically or electromyographically.4 Earlier TOF ratio (TOFR) of ≥ 0.7 was considered gold standard, however later TOF ratio of >0.75 was used as this is associated with the ability to raise the head for five seconds, open eyes widely, cough, protrude the tongue and protect the integrity of the airways.4,5 Now a days satisfactory recovery of neuromuscular function is defined as a TOF ratio 0.9.6
The potential for neostigmine to rapidly restore neuromuscular function is limited. It will not effectively antagonize 100% neuromuscular blockade and requires 10 min for peak effect. Even when the TOF ratio has recovered to unity, the majority of acetylcholine receptors may still be occupied by NMB, potentially rendering a patient susceptible to muscle strength fatigue with hypothermia or respiratory acidosis. Fade in the twitch response occurs after tetanic stimulation of a neuromuscular unit that has recovered from neuromuscular blockade. This observation supports the notion that even recovery to a TOF ratio 1.0 is not a true baseline and all patients who receive a NMBA should receive anticholinesterases for its reversal.7 However, excessive neostigmine may potentially cause neuromuscular block in a number of ways:
In our practice, vecuronium bromide is the most commonly used neuromuscular blocking agent for general anaesthesia. Generally we reverse the residual effect of any NMBA at the end of the surgery with 40-50 µg kg-1of neostigmine. Since there is paucity of literature on low dose neostigmine for reversal of residual neuromuscular blockade, we conducted this study to find out the most appropriate dose of neostigmine for adequate reversal of neuromuscular blockade.
This prospective randomized, double blind study was conducted in our Institution (department of Anaesthesiology and Critical Care) over a period of six months. One hundred and twenty adult patients, of either sex, between 20-40 years of age, belonging to ASA I and II were enrolled for this study. Patients undergoing operative procedures (duration lasting from 90-120mins) requiring tracheal intubations were included in the study. Informed and written consent was obtained from all the patients (Table 1).
Type of Surgery |
Group A |
Group B |
Group C |
Group D |
Upper abdominal surgery |
6 |
7 |
5 |
6 |
ENT Surgeries |
5 |
6 |
7 |
4 |
Neurosurgery |
4 |
5 |
4 |
3 |
Lower abdominal surgeries |
5 |
4 |
6 |
5 |
Breast surgery |
4 |
6 |
4 |
5 |
Others |
6 |
2 |
4 |
7 |
ASA I |
22 |
24 |
20 |
23 |
ASAII |
8 |
6 |
10 |
7 |
Table 1 Table showing inclusion criteria (types of surgeries and ASA grading) in different groups
Exclusion criteria
Patients with:
linical evaluation
All the patients were subjected to detailed clinical examination and routine biochemical investigations in order to rule out any systemic illness.
Preparation of the patient: Patients were kept fasting for six hours prior to surgery and received premedication in the form of tablet alprazolam 0.25 mg and tablet ranitidine 150 mg the night before and two hours prior to surgery with sips of water.
Anaesthesia technique: On arrival in operating room all the necessary monitors (electrocardiography (ECG), noninvasive arterial blood pressure (NIBP), pulse oximetry (SPO2), temperature and neuromuscular monitoring) were attached to the patients. Baseline values of heart rate, blood pressure and oxygen saturation were obtained and recorded. Intravenous cannulation was done with 18G cannula and Ringer lactate solution started. Induction of anaesthesia was carried out with glycopyrrolate (0.2mg), fentanyl (2 µg kg-1) followed by sleep dose of propofol (2.0-2.5 mg kg-1) IV. At this stage, neuromuscular monitoring was done with acceleromyography (TOF Watch SXR; Organon) with the acceleration transducer fixed on volar aspect of distal phalanx of thumb. Surface electrodes were placed over the course of ulnar nerve, just proximal to the wrist crease, on the forearm on the side other than used for monitoring of blood pressure and intravenous infusion. Acceleromyography was calibrated using the preprogrammed TOF-Watch calibration mode applying supramaximal stimulation current. Supramaximal current is the current above which there is no significant increase in electromyographic amplitude. When the TOF-Watch is switched on, it automatically sets the current to 60mA and amplitude to 100%. The current is then reduced in decrements of 5 mA till response of ≤ 90% is reached. Then 10% is added to next higher value and this current is taken as supramaximal current.
After stable base line recording of the TOF response, vecuronium bromide 0.1mg kg -1 IV was administered to facilitate tracheal intubation. Capnometer was attached at this stage. During surgery, vecuronium bromide infusion was used to maintain TOF response to two twitches. Anaesthesia was maintained with 33% oxygen in nitrous oxide along with sevoflurane 2-3% to maintain MAC of 1 till the end of the surgery. Intermittent boluses of injection fentanyl 0.5 g kg-1 were given every 30 min. End tidal concentration of CO2 was maintained between 32 and 36 mmHg and central body temperature between 35-37 oC throughout the surgical procedure.
Vecuronium bromide infusion was stopped at the end of the surgery. Once TOFR recovered to 0.4, all patients received injection glycopyrrolate 0.01mg kg-1 and neostigmine as per the group assigned. Depending on the dose of neostigmine used for reversal, all the patients were randomly allocated to one of the following four groups with the help of computer generated tables:
Group A (n=30) will receive neostigmine 10 µg kg-1
Group B (n=30) will receive neostigmine 20 µg kg-1
Group C (n=30) will receive neostigmine 30 µg kg-1
Group D (n=30) will receive neostigmine 40 µg kg-1
In order to ensure double blinding, a total solution of 12 ml of reversal was prepared for all the patients by an assistant and the observer would not know the dose of neostigmine used in a particular patient and extubation was done as per standard clinical practice. Recovery time was recorded from neostigmine administration to TOF ratio=0.7 and TOFR ≥ 0.9. However, recording of TOF ratio was continued for another 10 min at one min interval. Further readings were taken at 30 min and 60 min duration.
Following data was recorded-neuromuscular monitoring: TOF ratio was recorded at every 15 seconds after the administration of neostigmine till TOF ratio is ˃0.7 and >0.9 and for another 10 min at 1 min interval after TOF ≥0.9. Further readings were taken at 30 min and 60 min. Time of recovery was recorded from time of neostigmine administration to
Heart rate and blood pressure
Side effects (in respect to time of occurrence and number of episodes) such as desaturation, upper airway obstruction, arrhythmias, bradycardia, nausea, vomiting, bronchoconstriction and salivation were recorded.
The relevant data of each patient from the time of neostigmine administration was noted and entered into the patient proforma (Appendix). Data was compiled and analyzed statistically using ANOVA, Chi-square test, Turkey Kramer Multiple Comparison test and Student t-test.
Recovery was fastest in group D and there was statistically significant difference in recovery time with other groups. Group B and group D also had reasonably fast and sustained recovery. Recovery time in group B (3.65±0.48 min) is significantly less than that in group in group A (4.68±0.66 min) with p < 0.001. Recovery in group C was significantly faster (3.05±0.34 min) than group B with p < 0.001 which in turn was slower than group D (2.16±0.41 min) with p value < 0.001.
Group D had fastest recovery, but it was not sustained and there were two cases in which there was fall in TOFR once adequate recovery was achieved. In group A, B and group C, there were no cases with fall in TOFR once it was recovered to 0.9 i.e. the recovery was sustained. In group D (patients receiving 40 µg kg-1) there were 6.6% of cases (2 out of 30) in which recovery was not sustained and Recurarization was observed. Seventy percent patients in group A had adequate recovery with in 5 min where as in other groups all the patients had complete recovery with in 5 min. All the patients had adequate recovery with in 10 min. There were no significant differences in all the groups regarding episodes of bradycardia, junctional rythms or arrhythmia, hypotension or hypertension after any dose of neostigmine. Table 2 showing time required for recovery (min.) of TOFR in different groups (Graph 1 & 2).
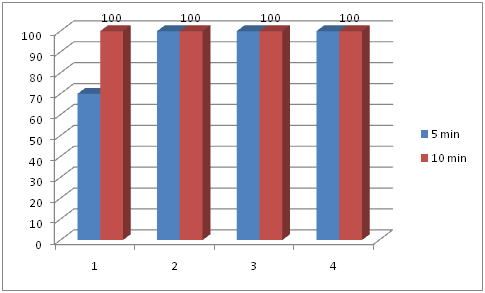
Graph 2 Probability of successful reversal (TOFR ≥ 0.9) within five and ten minutes after different doses of neostigmine.
Group |
Time (minutes) Mean ± S.D. |
A |
4.68±0.66 |
B |
3.65±0.48 |
C |
3.05±0.34 |
D |
2.16±0.41 |
Table 2 Time required for recovery (min.) of TOFR in different groups
Figure
We wanted to evaluate the ability of reduced doses of neostigmine to facilitate recovery from shallow degrees of residual paralysis (TOF ratio= 0.4) by vecuronium. Our study shows that neostigmine in doses of 10–30 µg kg-1 are sufficient in antagonizing shallow residual block at the end of the surgery. As little as 20 µg kg-1 of neostigmine may successfully reverse it within 5 min.
To antagonize moderate neuromuscular block corresponding to 1–3 TOF responses, 40 –70 µg kg-1 of neostigmine is required. For shallower but still potentially harmful degrees of residual paralysis, there is still no dosing recommendations.9 Findings of Debaene et al.4 are of interest in this context who reported that even after 2 h of a single dose of vecuronium about 30% of patients still had shallow degree of residual paralysis. This might raise the question of routine reversal based on one single standard dose of anticholinesterases (neostigmine 2.5 mg), which may result in inappropriately high neostigmine doses, in some patients, for the actual degree of residual paralysis.4 According to the findings of Caldwell, high dose neostigmine may increase weakness rather than improve neuromuscular recovery.10 Unfortunately, the neostigmine requirements to reverse these shallower degrees of residual paralysis have not yet been determined, at least not when applying the current criteria for adequate neuromuscular recovery (i.e., a TOF ratio ≥0.9). Since the most important neuromuscular functions are pulmonary ventilation, airway protection and maintenance of airway patency, ideally, recovery in these muscles should be measured directly. A reasonable and practical alternative is to assess muscle groups (including eyes, face, neck, skeletal muscle) in which recovery is consistently preceded by that in the diaphragm and muscles of the upper airway. At TOF ratios < 0.90 vecuronium induced partial paralysis causes pharyngeal dysfunction and increased risk for aspiration. The upper esophageal sphincter muscle is more sensitive to vecuronium than the pharyngeal constrictor muscle.11
Complete reversal of residual blockade depends upon the time elapsed between last administration of NMBA and reversal agent and also on the degree of block at the time of reversal. Anticholinesterases also exhibit a ceiling effect when used in larger doses. This is evident with the administration of a second dose of neostigmine (0.07 mg kg-1) which results in no further recovery from profound neuromuscular block. The fixed neostigmine (40-70 mcg/kg) dose may be explained by its ceiling effect.12 Thus, the question arises whether neostigmine still antagonizes moderate degrees of residual paralysis (i.e.1–3 TOF counts) or whether higher pre reversal degrees of neuromuscular blocks are required.13
If no TOF fade is detected with a simple nerve stimulator, then the TOF ratio is at least 0.4 but it may also be 0.9 or 1.0. In our study, we aimed to find out an appropriate dose of neostigmine for the reversal of shallow residual neuromuscular block (TOFR=0.4). The time to recover from a TOF ratio of 0.4 to ≥ 0.9 was reduced from 4.6 min (10mcg/kg of neostigmine) to 2.1 min when 40 µg kg-1 of neostigmine was used (Table 2). However, the dose of neostigmine actually needed depends on the pre reversal degree of neuromuscular block. High doses of 40 µg kg-1 of neostigmine might have paradoxical effects. This has to be considered when the recommendation of a neostigmine dose to reverse shallow neuromuscular block is based on the result of a simple nerve stimulator.
According to the results of the present study at least 20 µg kg-1 of neostigmine should be given to reverse a TOF ratio of 0.4 within 5 min. Indeed, taking a TOFR of 0.9 as the endpoint of adequate neuromuscular recovery, the probability of success was 100% after the administration of 20 µg kg-1 of neostigmine, when compared to TOFR of 0.4. In the group receiving 10 µg kg-1 of neostigimine, 21 patients out of 30 recovered to TOFR of 0.9 (the probability of success was 70%). In the remaining 9 patients recovery was complete within 10 min. Neostigmine when given in 30µg kg-1 and 40µg kg-1 dose led to complete recovery from neuromuscular blockade within 5 min. Moreover, all the 120 patients even reached a TOF ratio of 1.0 within the 10min time interval. In view of these results, clinically relevant consequences of residual paralysis are unlikely after all the doses of neostigmine investigated (Graph 2).
Recovery in group receiving 40µg kg-1 was significantly faster than group receiving 10 µg kg-1 (2.16 min v/s 4.68 min). Recovery in groups receiving 20 and 30 µg kg-1 neostigmine respectively was also considerably fast (3.65 v/s 3.05 min). Although recovery in group D patients was significantly faster than all other groups, there were two patients in which there was significant fall of TOF ratio once it recovered to ≥ 0.9.This is a very significant finding. In one patient, after giving neostigmine in a dose of 40 µg kg-1 the TOF ratio recovered to ≥ 0.9 with in 2 min and then there was a fall in TOFR to 0.72 in the next 1 min.This TOFR was sustained for further eight readings taken 15 sec apart (2 min.) and it recovered to ≥ 0.9 in 2 min 15 sec. In another patient of the same group, the recovery time was 1 min 15 sec. Following recovery there was a fall in TOFR to 0.76 in 3 min which was sustained for 90 sec. This finding has a high statistical significance as around seven percent of the patients had fall in TOFR after adequate recovery. This was seen only in group D (Graph 1).
Although reversal was sustained with a dose of 10 µg kg-1 and there was no fade of TOFR once adequate recovery was established, but the time of recovery was significantly higher than other doses of neostigmine. Time of recovery is very important as this is the time during which many untoward complications can occur. It was for this reason that an attempt was made to identify a smaller dose (20 µg kg-1) of neostigmine which would produce adequate antagonism of residual block without increased fade.11
Fuchs-Buder et al.14 conducted a similar study to investigate the dose–effect relationship of neostigmine to antagonize residual paralysis after atracurium blockade where desflurane/propofol were used for maintainance.14,15 They supported that reduced doses of neostigmine (10–30µg kg-1) antagonized shallow atracurium block. In our study, neostigmine was given in the doses of 10, 20, 30, and 40 µg kg-1 at a TOFR of 0.4 and we considered the end point of adequate recovery as TOFR ≥0.9. Fuchs-Buder et al.14 reported that when given at a TOF ratio of either 0.4 or 0.6, time to 0.9 and 1.0 TOF ratio was significantly shorter with any dose of neostigmine than without. All the patients of our study recovered to TOFR ≥ 0.9 within 10 min of neostigmine administration and the recovery times of all patients are significantly less than the above study. Though the reasons for the difference in recovery times are not very clear it could be due to the continuous propofol infusion/desflurane and sufentanil used for maintenance of anaesthesia in Fuchs et al. study which may have potentiated the neuromuscular blockade.
We have given reversal only at a TOFR of 0.4 and not at 0.6. Reversal given at various levels of neuromuscular recovery may have clinical implications. Routinely reversal is given depending on the start of clinical recovery (without neuromuscular monitoring). Administration of reversal agent at deep stage of blockade will lead to delayed recovery and the drug requirement will also be more. This is of particular importance in patients where adverse effects of neostigmine are highly undesirable (patients with bronchoconstriction and bradyarrythmias) and the dose needs to be adjusted.
Various factors may have influenced the outcome such as age, sex, weight. But these variables were comparable in all the four groups of our study. The number of patients chosen for the study was based on the primary outcome. So we were unable to study the other outcomes such as various levels of pre reversal block to be antagonized by neostigmine, at what value of TOFR should the reversal start and whether influence of volatile agents may have potentiated the block. Further studies need to be carried out to find that this small dose of neostigmine (20mcg) may equally be effective for other muscle relaxants.
Small degree of residual bock is both difficult to detect and potentially harmful. Normal doses of neostigmine may produce paradoxical weakness. Adequate recovery from neuromuscular blockade especially after prolonged surgeries is still a challenge with respect to both dosing regimen of anticholinesterases and the ideal TOFR at which complete reversal occurs. This study shows recovery is fast with both 20 and 30 µg kg-1 of neostigmine, (3.65 min v/s 3.05 min) and there are no side-effects with both the doses so we suggest our readers that 20 µg kg-1 could be the appropriate dose for reversal of residual neuromuscular blockade.
None.
The authors declare no Conflicts of interest.

©2016 Kajal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.