eISSN: 2574-9838


Research Article Volume 3 Issue 4
Department of Physical Medicine & Rehabilitation, University of Missouri, Columbia
Correspondence: Carmen M Cirstea, Department of Physical Medicine & Rehabilitation University of Missouri, School of Medicine, Columbia, Tel 573-884-8792
Received: June 27, 2018 | Published: July 6, 2018
Citation: Cirstea CM. The role of spared spinal cord in post-surgery recovery depends on age in cervical spondylotic myelopathy–a case series study. Int Phys Med Rehab J. 2018;3(4):287-293.. DOI: 10.15406/ipmrj.2018.03.00117
Object: New evidence shows that the degree of microscopic alterations in spinal cord (SC) regions appearing normal on conventional MRI has some predictive value of neurological impairment and recovery in patients with cervical spondylotic myelopathy (CSM). Although SC physiological reserves and plastic potential are likely to decrease as a function of age, the effects of age on such relationships remain unknown. We hypothesized that in CSM patients the preoperative level of upstream, propagating degenerative processes within spared SC from the injury site projects the postoperative functional outcome and this relationship depends on the patient’s age at the diagnosis.
Methods: Nine CSM patients exhibiting neurological symptoms less than one year underwent decompressive surgery. Preoperative neuronal (N-acetylaspartate, NAA) and cellular turnover (choline, Cho) biomarkers were quantified by means of MR spectroscopy (MRS) in spared SC regions in the closed proximity to the compression level (C2 level). Pre- and postoperative (at six months after surgery) clinical statuses were assessed using modified Japanese Orthopaedic Association (mJOA) and 9-Hole Peg test (9HPT). Relationships between MRS biomarkers and clinical changes were determined and controlled for patient’s age.
Results: Consistent with prior findings, significant clinical improvement was detected postoperative (mJOA, p=0.002; 9HPT, p=0.001). As predicted, NAA and NAA/Cho significantly related to changes in mJOA (p=0.02) and 9HPT (p=0.005) scores respectively. Notably, these relationships did not survive age correction (p=0.1 and p=0.06 respectively).
Conclusions: Our preliminary results are consistent with MRS potential to assay the degree of microscopic injury and viability of the spared SC to prognosticate functional recovery after decompressive surgery in this population. Likewise, our findings provide initial evidence that the contribution of the spared SC in postoperative recovery depends on the patient’s age at the diagnosis.
Keywords: cervical spondylotic myelopathy, MR Spectroscopy, spinal cord, decompressive surgery, clinical outcome, age
Cervical spondylotic myelopathy (CSM) is the most debilitating form of degenerative spinal disorder1 with a treatment cost running in billions of dollars each year in the United States.2 Considering that in 2050 the proportion of the population over 60 years is projected to double,3 clinicians will be required to manage an increasing number of CSM cases. Clinically, CSM may present abruptly or insidiously with neurologic dysfunction of varying severity. The symptoms might improve, stabilize, or worsen. For the later scenario, decompresssive surgery is often performed. Yet, the response to surgery significantly varies among patients.4 Further, the surgery itself can carry significant risks, particularly in the elderly population.5,6 Considering these, there is a major interest in determining the patients that are most likely to obtain clinical benefit from surgery and avoid exposure to surgery-associated risks for those that are unlikely to improve. At present, there are no reliable and accurate markers in predicting response to surgery in these patients.7 For example, the gold-standard MRI markers, i.e., spinal cord (SC) compression, SC signal change, reach Class III level of evidence.8 This failure may emanate from the fact that these markers provide excellent macroscopic anatomical detail, yet extremely limited specific information regarding the SC microstructure and function. For instance, some patients may not demonstrate functional improvement after surgery despite MRI findings of macroscopic SC recovery. It is likely that in such patients there are microscopic changes affecting SC function that are not revealed by standard MRI. As a result, there is an increasing interest in the utility of advanced MRI techniques that can provide such information.9,10 Diffusion tensor imaging (DTI) has been recently used to quantify SC tracts microstructure in this patient population11–14 and demonstrated that axonal disruption at the level of maximal SC compression is moderately associated with pre- and postoperative dysfunction (as evaluated by modified Japanese Orthopaedic Association, mJOA).15 Importantly, when other clinical tools than mJOA have been employed, different results were found.16 This infers that the effects of SC injury on axonal status may not occur only at the most compressed level and provides further support that remote microscopic changes within SC could play a critical role in recovery.
Magnetic resonance spectroscopy (MRS) is the other proposed technique to may provide insights into remote neural microscopic/cellular changes associated with CSM.4,17 This technique quantifies biomarkers related to neuronal and glial cell integrity, inflammation/necrosis, excitability/inhibition, and intracellular metabolism in brain or SC regions appearing normal on conventional MRI.18 Our recent work has demonstrated that lower levels of a neuronal biomarker (N-acetylaspartate, NAA), which is generally attributed to neuronal loss and/or metabolic dysfunction,19 in the motor cortex controlling arm function are related with a reduced probability of successful postoperative recovery in these patients.20 While this work focused on brain, here we directed our attention on microscopic alterations in radiological normal appearing SC regions in the closed proximity to the compression site. At SC level, the feasibility of using MRS biomarkers to investigate the extent and severity of SC damage has been demonstrated.21,22 Critically, these biomarkers have been also histopathological validated as a measure of SC damage.23 Prior SC MRS studies in CSM all reported lower levels of NAA and higher levels of an indicator of cellular turnover (choline, Cho17) in spared SC that correlate with neurological impairment.24–27 A degenerative process which propagates upstream from the injury site (or retrograde degeneration)28 was thought to cause NAA and Cho changes in these patients. Similar findings have been reported in other SC pathologies, such as multiple sclerosis,29 amyotrophic lateral sclerosis,30 providing further support for such degeneration process. However, to our knowledge, there exists only one other published study using SC MRS to investigate the relationship between these biomarkers and recovery from impairment after surgery in this patient population.31 Holly et al. found that preoperative levels of NAA and Cho were predictive of neurological outcome at one year after surgery. This result was interpreted as evidence for the possible role of spared SC in postoperative recovery. However, the physiological reserves32,33 and plastic potentia34 of the SC decrease as a function of age and, as stated above, CMS is mainly prevalent in elderly. Therefore, the question of whether age influences the role of the spared SC in recovery remains unknown. Nevertheless, the relationships between preoperative NAA/ Cho and recovery post-surgery in this initial report is suggestive of possible relationship between functional normalization of the microscopic damaged SC and recovery from impairment and serves as an important motivation for the current study.
Here, we assessed whether preoperative degree of microscopic injury and viability of the spared SC (quantified by NAA and Cho levels) prognosticates functional recovery at six months postoperative in nine patients. The six-month assessment has been selected based on the evidence that at this time postoperative patients usually reach neurological stability.6 We hypothesized that patients with less cellular alterations (higher NAA, lower Cho) rostral to the compression level would have greater postoperative recovery. In addition to the primary hypothesis concerning NAA and Cho, we tested a secondary prediction involving myo-inositol (mI) which is a precursor of the phosphatidylinositol second messenger system prevalent in glial cells.35 On the basis of the augmented expression of neurotrophins in glial cells within the SC regions rostral to the level of compression,36 we predicted that the higher levels of mI would be related with higher neuronal survival and remyelination and consequently to an increased probability of successful postoperative recovery. We then adjusted these relationships for differences in age at the diagnosis. If they would not survive the correction, we then advocated that the contribution of the spared SC in postoperative recovery depends on the patient’s age at the diagnosis.
Participants’ characteristics
Written informed consent in accordance with the Office for the Protection of Research Subjects at Bagdasar-Arseni Hospital has been obtained from all participants before enrollment. Nine CSM patients (five men, mean±SD age, 60±14 years, (Table 1)) were included if they exhibited arm weakness and/or gait abnormalities less than one year and had experienced recent clinical deterioration that led to the decision for surgery. The patients have been excluded if they had history of previous cervical surgery, SC compression at C2 level or cervicomedullary junction, other neurological/neuromuscular/orthopedic problems, substance/alcohol dependence, and MRI contraindications. There was no gender, racial, or handedness exclusions (Table 1).
Sex |
Age (yrs) |
Compression level |
MRI altered |
Surgical approach |
mJOA |
9HPT (s) |
||
pre |
post |
pre |
post |
|||||
F |
68 |
C4 - C7 |
- |
P |
17 |
18 |
16.5 |
16.5 |
M |
65 |
C4 - C6 |
- |
P |
15 |
17 |
97.5 |
61.5 |
F |
62 |
C4 - C5 |
- |
A |
13 |
16 |
24 |
23 |
F |
63 |
C4 - C6 |
- |
P |
13 |
15 |
20 |
19.5 |
M |
63 |
C3 - C7 |
+ |
P |
11 |
13 |
32.5 |
29 |
F |
77 |
C4 - C5 |
+ |
P |
11 |
16 |
16 |
15.5 |
M |
44 |
C5 - C6 |
+ |
P |
11 |
13 |
23.5 |
22.5 |
M |
66 |
C4 - C6 |
+ |
P |
10 |
11 |
19.5 |
19 |
M |
31 |
C5 - C6 |
+ |
A |
7 |
13 |
23.5 |
23 |
Table 1 Demographics, clinical and MRI characteristics, and surgical approach
M, male; F, female; yrs, years; s, second; +/- with/without intramedullary alterations; A, anterior, P, posterior; mJOA, modified Japanese Orthopedic Association, normal=18; 9HPT, 9-Hole Peg test, averaged performances for each hand (s); pre, before surgery, post, at six months after surgery.
Surgical management
All surgeries were performed by S.C.C. The choice of surgical technique was based on the location of compressive pathology, number of compression levels, and spinal alignment and stability. Anterior discectomy with fusion was performed in 22% of the cases, and posterior laminectomy with fusion was performed in the rest of the patients (Table 1). None required a combination anterior-posterior surgery. There were no intra- (nerve injury, hemorrhage) or immediate postoperative (hematoma, infections, dysphagia, pain) complications. None of the patients presented neurological deterioration at six months postoperative.
Study protocol
Clinical assessments were performed prior to and at six months after surgery. Two valid and highly reliable tests were administrated: mJOA, a frequently used clinical/research tool in this population,37 assessing motor and sensory function of upper and lower extremities and sphincter function (normal=18), and 9-Hole Peg test (9HPT),38 evaluating fine hand motor coordination (Rolyan 9-Hole Peg Test Kit, model A8515; Sammons Preston, Division of Patterson Medical, IL, USA). For later, we scored the amount of time it takes to place and remove 9 pegs; two attempts were allowed for each hand and the best performance for each hand was considered; the performances for both hands were averaged to avoid confounding effect of handedness and unequal disability between hands. Clinical assessments were carried out by one physical therapist with more than 10-year experience in this patient population. The clinical assessment duration was about 35 min. Table 1 shows patients’ clinical information prior to surgery: eight patients were noted to have motor/sensory changes in the arms and four had weakness in the legs. Overall, the patients presented with moderate neurological impairment (mJOA, 12.0±2.9).
Because preoperative clinical status may affect the postoperative outcome,7 recovery was defined with respect to this: recovery rate for mJOA (RR=(mJOApost-mJOApre)*100/(18-mJOApre)), normalized change for 9HPT (NC=(9HPTpost-9HPTpre)*100/ 9HPTpre).
MRS studies, consisted of structural MRI and MRS of the cervical spine, were performed prior surgery on a 1.5-T Siemens Magnetom Avanto system, using the lower elements from the head coil and all channels from the neck coil (Medinst Imaging Center). Clinical MRI scans consisted of thin-sliced T2-weighted sequences in the sagittal plane (TE/TR=125/1500ms, FOV=200mm, voxel size=0.6x0.6x0.6mm). All patients had MRI evidence of stenosis, such as spinal canal narrowing related to advanced cervical spondylosis. Five of the patients had SC hyperintensity and the other four had normal signal intensity throughout the SC (Table 1). None of our participants exhibited other spine pathology. An additional T2-weigthed sagittal MRI (TE/TR=89/3600ms, FOV=220mm, voxel size 0.8x0.6x3mm) was acquired to localize spectroscopic voxel. Pulse-gating point-resolved spectroscopy (PRESS, TE/TR=30/ 1500ms, averages=200, flip angle 900, spectral width=1000 Hz) with chemical shift selective water suppression was used. A spectroscopic voxel with dimensions of 10mm (right-left), 10mm (anterior-posterior), and 30mm (cranial-caudal) was placed along the main axis of SC at the C2 level with the caudal limit placed on C2/C3 intervertebral disc (Figure 1A). The anatomical locations of the spectroscopic voxels were similar in all patients. Six saturation bands were placed around the voxel to minimize cerebrospinal flow (CSF) artifacts and suppress signals from outside the voxel. Automated/manual shimming was performed to achieve an optimal full width at half maximum of<20 Hz of the water signal. Patients were instructed to avoid swallowing during MRS acquisition to prevent motion artifacts. Likewise, infrared finger pulse oximeters with gating delay of 400ms after cardiac electrical systole<39 was employed to reduce the influence of CSF flow and SC motion.

Figure 1 (A) Location of the spectroscopic voxel at the level of the C2 vertebral body on sagittal (top row) and coronal (bottom) T2-weighted image of one patient (Patient #3, male, 66 years old, Table 1). (B) LCModel output derived from the voxel shown in A. ppm, parts per million; NAA, N-acetylaspartate; Cho, choline; mI, myo-inositol; A, anterior, P, posterior, L, left, R, right.
MRS data were processed by means of LCModel40 at Kansas University Medical Center. Implementation of short echo time during acquisition allowed us a clear identification of our metabolites of interest: NAA, Cho, mI. Creatine (Cr) signal was used as a reference signal41 and ratios of our metabolites of interest were calculated with respect to Cr using LCmodel algorithm fitted to experimental data in the frequency domain. Ratios between our metabolites of interest, i.e., NAA/Cho, NAA/mI, Cho/mI, were also explored (Figure 1).
Statistical analysis
The analysis focused on MRS variables (preoperative NAA, Cho, mI ratios) and clinical outcomes (preoperative mJOA, 9PHT; RR-mJOA, NC-9PHT). Spearman Rank Order correlation test was used to determine the relationships between variables and outcomes. The effects of each variable on outcomes have been studied when patient’s age is controlled for. Pre- and postoperative clinical scores were also compared using two-tailed paired Student’s t-test. Analysis was performed on SPSS 24.0 (Chicago, IL, USA) and significance level was set at p<0.05.
Preoperative C2 MRS variables
Signal-to-noise ratios for all spectra were within recommended tolerances and the Cramer Rao bounds of all metabolites were<20 in all participants reflecting high spectral resolution43 and allowing reliable quantification of our metabolites of interest (Figure 1B).
Consistent with prior SC MRS findings in CSM, 24–27 mean NAA/Cr ratio was 1.52±0.57 with a range between 0.79 and 2.49. The values of mI/Cr and Cho/Cr also corroborate well with those reported by others:24–27 1.93±0.60, range from 1.37 to 2.92 for mI/Cr and 0.53±0.12, range from 0.35 to 0.67 for Cho/Cr respectively. We also evaluated NAA/Cho to estimate concurrently both the neuro/axonal integrity and the amount of cellular turnover and the values reported here, 2.82±0.58, range from 1.76 to 3.74, are also consistent with the literature.24–26 There were no significant correlations between age and any metabolite ratio (p varies between 0.07 to 0.9).
Postoperative clinical improvement
In accordance with previous studies (see review42), we found significant clinical improvement at six months after surgery. mJOA score improved significantly from 12.0±2.9 to 14.7±2.3 (RR-mJOA=51.4±26.8%, range from 12.5 to 100%, p=0.002), suggestive of a better neurological status. Significant improvement has been also reported in 9PHT (from 30.3±25.7s to 25.5±14.1s, p=0.001, NC-9PHT=-7.4±11.5%, range from 0 to -36.9%), reflective of a better hand function. Please note that age was not significantly related to any clinical score (p between 0.2 and 0.5).
Preoperative C2 NAA/Cr and NAA/Cho correlate with postoperative clinical improvement surgery but these correlations did not survived age correction
Our next series of analysis investigated whether C2 metabolites are related to the clinical improvement stated above. As predicted, NAA/Cr ratio was significantly correlated with RR-mJOA (r=0.75, p=0.02, (Figure 2A), (Table 2). In addition, NAA/Cho ratio was significantly related to changes in 9HPT scores (Figure 2B) (Table 2) r=0.83, p=0.005). These findings are consistent with the only one other published study using MRS in similar population31 showing a significant relationship between the preoperative C2 neuronal dysfunction/cellular turnover and clinical outcome at one year post-surgery. Notably, Cho/Cr did not significantly correlated with clinical improvement (p between 0.2 and 0.9). This could be due to the fact that this ratio assays only the amount of cellular injury/turnover and it might not be as predictive of outcome as the combination of neuronal damage and cellular turnover as assayed by NAA/Cho ratio. Contrary to our expectations, there were no statistical relationships between mI/Cr ratios and clinical improvement post-surgery (p between 0.2 to 0.9). This supports the idea that this marker may not be sensitive enough to capture the glial changes with recuperative potential. Alternatively, it is possible that the glial changes at this level are not likely significantly involved in functional recovery quantified here. We also explored the relationships between NAA/mI and Cho/mI and clinical improvement and found no significant relationships (p between 0.4 and 0.8). Obviously, more work in larger samples is needed.
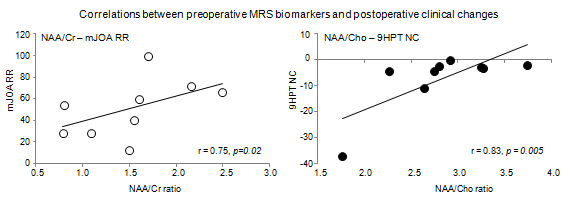
Figure 2 Scatter plots showing the relationships between preoperative N-acetylaspartate (NAA)/Creatine (Cr) ratios and the rate of recovery (RR) of mJOA (left panel) and N-acetylaspartate (NAA)/Coline (Cho) ratios and the normalized change (NC) of 9HPT (right panel).
Preoperative variables |
Preoperative values |
RR mJOA |
NC 9HPT |
|||
r |
p-value |
r |
p-value |
|||
NAA/Cr |
1.5±0.6 |
0.75 |
0.02* |
-0.46 |
0.21 |
|
Cho/Cr |
0.5±0.1 |
0.50 |
0.17 |
-0.11 |
0.77 |
|
mIn/Cr |
1.9±0.6 |
0.47 |
0.20 |
-0.12 |
0.76 |
|
NAA/Cho |
2.8±0.6 |
0.62 |
0.07 |
0.83 |
0.005* |
|
mJOA (#) |
12.0±2.9 |
0.61 |
0.08 |
-0.44 |
0.23 |
|
9HPT (s) |
30.3±25.7 |
-0.45 |
0.22 |
-0.79 |
0.01* |
|
Age (yrs) |
59.9±13.8 |
0.42 |
0.25 |
-0.21 |
0.58 |
|
Table 2 Spearman correlation coefficients (r) and p-values between preoperative variables and clinical changes
*signifies p<0.05, mJOA, modified Japanese Orthopedic Association, normal=18; 9HPT, 9-Hole Peg test, averaged performances for each hand (s); RR, recovery rate; NC, normalized change; yrs, years; s, seconds.
As expected, the correlations between preoperative MRS biomarkers and postoperative recovery did not survive age correction (p=0.1 and p=0.06 respectively). This could be due to the well-reported age-related changes in SC physiological reserves and plastic potential.32–34
Preoperative mJOA did not but 9HPT did correlate with postoperative clinical improvement
In contrast with a recent multi-center study, the relationship between preoperative mJOA and its change with surgery did not reach statistical significance (r=0.61, p=0.08) in our sample. Importantly, the magnitude of correlation coefficient indicates that in a larger sample size is probably to reach significance. In contrast, the preoperative 9HPT significantly correlated to its six-month change (r=-0.79, p=0.01) even in this small sample. This indicates that incorporation of a quantitative, detailed, and specific deficit assessment, such as 9HPT, may provide a richer and more objective characterization of functional impairment and therefore a better outcome prediction compared to crude clinical tools, such as mJOA. Please note that mJOA is a regularly assessment tool employed in prediction studies in this population.
Patient’s age did not predict postoperative clinical improvement
Age was deemed an insignificant predictor of clinical changes in our sample (p between 0.2 to 0.8). This finding is in contrast with prior reports, although not universally accepted, demonstrating that patient’s age is a major predictor of surgical outcome25. It is possible that this is due to our small sample size.
Preoperative C2 NAA/Cho correlated with preoperative clinical impairment
In agreement with prior studies,26,28 NAA/Cho ratios were significantly correlated with the preoperative mJOA and 9HPT (r=0.76, p=0.018 and r=0.72, p=0.03). These findings imply that greater cellular alterations (lower NAA/higher Cho) proximal to the injury site, probably due to retrograde degeneration processes,44 are associated with worse impairment. Similar to those described above, these correlations did not survive age correction (p=0.051 and 0.078 respectively), suggesting that these relationships are less important in elderly.
The correlations between other ratios, e.g., mI/Cr, Cho/Cr, NAA/mI, Cho/mI, and preoperative clinical status did not reach significance (p between 0.2 and 0.9), broadly agreeing with the quite selective nature for the neural events previously reported in this population.
We first predicted that MRS metrics of the spared SC provide important information regarding the potential to recover after surgery in this patient population. Our results demonstrated that all patients achieved functional improvement after surgery and this was significantly related to the preoperative levels of NAA/Cr and NAA/Cho. Patients with lower NAA (neuronal or axonal injury and/or neuronal metabolic depression) and elevated choline (increased cellular injury/turnover) had a poorer prognosis after surgery. We then predicted that these relationships will not survive age correction. Indeed, the relationships between functional improvement and preoperative levels of NAA/Cr or NAA/Cho became insignificant after age correction, raising the possibility that in older patients the contribution of the spared SC in recovery could be reduced. Possible reasons for this could be a decrease in the physiological reserve and/or neuroplastic potential of the SC as a function of age. Finally, no relationships were found between mI ratios and clinical changes, indicating that the effects are quite selective in nature. Below, we discuss these findings and their implications in detail.
Preoperative NAA/Cr and NAA/Cho correlated with postoperative clinical recovery but these correlations did not survive age correction
We hypothesized that the degree of microscopic injury and viability of the spared SC, as quantified by NAA and Cho, predicts the recovery potential for the next six months after spine surgery. We found that higher preoperative spinal cord NAA/Cr ratio was associated with higher mJOA scores. We also reported that NAA/Cho ratio is stronger and significantly related to the improvement in 9HPT score than the NAA/Cr ratio: a higher preoperative NAA/Cho was associated with greater improvement in time to execute the 9HPT after surgery. These findings are consistent with the idea that less microscopic alterations (higher NAA, lower Cho) at a level not directly affected by but closed to the injury are predictive of greater postoperative recovery. We assume that there is a spectrum of microstructural changes at this level24 that might normalize after surgery and ultimately contribute to functional recovery. These findings are consistent with the only one similar study in CSM31 and with a number of studies in other SC disorders29,30 implying that these MRS biomarkers reflect the neural recuperative potential at this level. Alternatively, we may consider these biomarkers as indicative of the newly developed compensatory pathways in the intact SC. However, our data cannot distinguish between these mechanisms and future research is needed to better understand the functional relevance of such remote SC microstructural changes. Nevertheless, these findings are clinically important and if proven reliable in larger samples, such understanding would be a significant advancement in the treatment of CSM patients. As mentioned above, a number of conventional or new MRI markers have been analyzed, yet none have been able to reliably correlate with neurological recovery.42 We advocate that the microcellular information provided by MRS in regions that appear normal on conventional MRI, providing data about distant microscopic effects of the SC damage, may advance our current understanding of why some patients recover while others no. This would be especially useful in CSM management.
As predicted, we found that these relationships did not survive the corrections for patient’s age at the diagnosis. As stated before, the predictive value of the patient’s age is still a debated subject.44,45 Even in our sample, the age by itself does not show a significant value in predicting postoperative clinical changes. However, when age was included in our model, the relationships between MRS biomarkers and postoperative recovery became insignificant. This is not surprising since our sample contains 78% patients older than 60. It is likely that in older patients the contribution of the spared SC in recovery post-surgery could be diminished and this is possibly due to the age-related reduction in SC physiological reserves (i.e., decrease in the number of motoneurons, anterior horn cells, and/or myelinated fibers in the corticospinal tracts and posterior funiculus32,33). Likewise, neuroplastic changes that take place at SC level is likely to diminish as a function of age39. However, we do not expect that the surgeons should discriminate on the basis of age but we suggest that older patients may exhibit some limitations in and/or different mechanisms underlying neurological recovery than younger patients.
Preoperative Cho/Cr or mI/Cr did not significantly correlate with postoperative clinical recovery
Contrary to our predictions, we failed to find significant correlations between Cho (alone) or mI and postoperative recovery. For Cho/Cr ratio, this could be due to the fact that this ratio is representative only for the amount of cellular injury/turnover and therefore it might not be as predictive of outcome as the combination between cellular turnover and neuronal damage, as assessed by NAA/Cho. For mI/Cr ratio, an explanation could be that this marker is no sensitive enough to capture the glial cells changes with recuperative potential. It is also possible that glial changes at this level are not likely significantly involved in the functional recovery. Indeed, there is evidence to suggest that oligodendroglia at the compression site, but not remotely, contribute to the demyelinating process reported in such patients.46 Obviously, further studies in larger samples are needed to better understand the molecular/cellular changes in spared SC and their functional relevance in this population.
Preoperative 9HPT but not mJOA correlated with postoperative clinical recovery
Preoperative 9HPT score, but not mJOA, again, the most used test in prognostic studies, significantly correlated with the clinical changes after surgery. This is not a surprising result. The mJOA score can be misleading due to the uneven weight placed on each component (upper-extremity sensorimotor, lower-extremity motor, and sphincter dysfunction) and hence misrepresenting the degree to which the SC and the specific white matter tracts are injured. Our results demonstrated that fine-grained clinical data, i.e., 9HPT for hand impairment, are more likely to identify stronger correlations between baseline focal neurological deficits and recovery. Thus, we advocate that using a broader array of clinical tests would probably give more power to show important correlations between clinical markers and outcomes in such patients.
Preoperative NAA/Cho correlated with preoperative clinical impairment but this correlation did not survive age correction
In agreement with prior studies,24,26 we found significant correlations between NAA/Cho and clinical impairment in the preoperative stage. Analogous to relationships between MRS biomarkers and postoperative clinical changes, these preoperative relationships did not survive age correction. These results are likely to provide support of this biomarker clinical relevance especially in younger population. Clearly additional effort is needed to recruit more patients to confirm our observation.
A small number of patients has been studied and future studies in larger samples are critically needed. SC MRS is technically challenging to use due to the small diameter of the SC and its mobility.21 To overcome some of these challenges we used i) the recommended voxel size and placement,39 ii) saturation bands to reduce CSF flow artifacts and suppress signals from outside the voxel, iii) pulse triggering to reduce the effects of CSF flow and spinal cord motion, and iv) instructed patients to stay still and avoid swallowing during acquisition. Due to the small diameter of the SC, the voxel included grey and white mater and both sides of the SC. This is important to note because grey matter, white matter, or the sides of SC may be differentially affected by the disease processes. Finally, other metabolites, such as glutamine/glutamate (Glx) and lactate, may be very informative. Glx has not been included due to high Cramer-Rao bounds while lactate has not been detected in our sample.
Only patients with symptoms no longer than one-year duration have been included. Because the patient became aware about his/her progressive disability late in the progression of disease, to find the exact duration of the symptoms was challenged.
We have previously described functionally relevant metabolite changes at the cortical level in these patients7. More studies are need to determine the relative contributions of different sites of plasticity and the conditions in which they apply differentially.
Despite the above limitations, our preliminary data provide evidence that SC MRS potentially offers an accurate method of predicting outcome in this patient population. Since MRS is increasingly available on clinical scanners with rapid acquisition and automated analysis, MRS-based biomarker is feasible in routine practice. Our findings are also consistent with the limited contribution of the spared SC in functional recovery postoperative in elderly. Clearly, more work is needed to evaluate its reliability.
This work was supported by EuroSpine–The Spine Society of Europe (TRF12-Eurospine to SCC and CMC). The Hoglund Brain Imaging Center is supported by a generous gift from Forrest and Sally Hoglund.
The author declares no conflict of interest.

©2018 Cirstea. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.