International Journal of
eISSN: 2475-5559


Research Article Volume 2 Issue 2
1The Institute of oil- and carbochemical synthesis, Irkutsk State University, Russia
1The Institute of oil- and carbochemical synthesis, Irkutsk State University, Russia
Correspondence: MA Lurie, The Institute of oil-and carbochemical synthesis, Irkutsk State University, Russia
Received: December 23, 2016 | Published: February 28, 2017
Citation: Lurie MA, Schmidt FK. Sulfur- and metal content, isotopic S and C- geochemical signs of oil genesis. Int J Petrochem Sci Eng. 2017;2(2):41-46. DOI: 10.15406/ipcse.2017.02.00030
Currently, a number of evidences witness the significant role of endogenic factors in oil and gas formation. It has been suggested that the components of endogenic fluids participate in the generation of oil systems. It is a common opinion that the most probable route of the formation of oils abiogenic part involves the reactions of oxides with hydrogen (Fischer-Tropsch synthesis).
This point of view as well as the notions on the origin of oil from bio-systems does not give clear understanding of the sources of oil heterocomponents, including, first of all, S, vanadium (V) and nickel (Ni). Stable correlations between concentrations of these components and other characteristics of oils allow one to conclude that sulfur and metals (which are contained in endogenic fluids) may play a certain role in oil formation.
In the present work we report that the interaction of endogenic CH4 and sulfur, owing to the ability of the latter to initiate condensation transformations of hydrocarbons, can lead to the generation of oil different type of hydrocarbons, sulfur-organic compounds and higher molecular structures such as asphaltenes and resins. In the course of these transformations, the metals aforementioned exert (due to their catalytic properties) various effects on hydrocarbon structures.
The data presented in the paper make it possible to explain some regularities of oil composition, which are poorly understandable from the positions of conventional views on oil genesis. Such regularities are as follows:
Isotopic transformation of carbon and sulfur, oils fraction, individual hydrocarbons and other natural systems are also discussed.
Over the last decades, many evidences have been found to support the theory of organic origin of oil and gas. At the same time, a number of facts indicating the effects of endogenic factors on oil genesis are growing. The oil origin should be considered as a result of interaction of two different flows of matter and energy. The sources of oil components should include not only organic matter (OM), mineral skeleton of natural reservoir and stratal waters, but also deep endogenic fluids.1Independently on the available opinions about oil origin, the researchers should explain the reasons causing the association of elements observed in oils. Are heteroelements, in particular S and metals, the “satellites” constituting the hydrocarbons system and imparting some specific properties to oil? Or these elements due to their reactivity play more significant role even on early stages of oil formation, thus determining its geochemical type? The latter assumption is supported by numerous evidences on stable direct correlations between concentrations of S, V + Ni, V, ratio V/Ni, concentration of aromatic compounds, asphalt-resin components, values of viscosity and density.2 Within the framework of organic theory of oil origin it is difficult to find an approach to explain stability of these correlations, especially when these characteristics are widely ranged.
The assumption of endogenic contribution to oil genesis prompts us to answer the question concerning the sources of carbon and heteroelements of abiogenic constituent of oil. Carbon could be found in endogenic fluids as carbon monoxide (CO), carbon dioxide (CO2) and methane (CH4). The widespread viewpoint about genesis of abiogenic oil suggests the possibility of hydrocarbons formation in the course of hydrogenation of carbon oxides by hydrogen, which is contained in deep fluids (Fischer-Tropsch synthesis). However, the proceeding of these reactions in earth crust is hindered by many reasons. The presence of hydrogen sulfide (H2S) and elemental sulfur (S0) in some endogenic gas mixtures3,4 inhibiting the Fischer-Tropsch reactions 5 is the principal obstacle for realization of these processes. Besides, like in the case of organic theory of oil origin, substantial difficulties are connected with understanding the reasons of hereocomponents (which concentrations are changed according to the stable regularities mentioned) appearance in oil.
On the basis of the existing notions on composition of endogenic fluids, containing along with hydrogen (H2), CO and CO2 a number of other components possessing reactivity, in particular, S0, we have put forward the hypothesis6 that the starting reaction of the formation of oil abiogenic constituent involves the interaction of CH4 and its nearest homologs with S0. It is known 7,8 that S0 initiates condensation reactions of CH4 and other hydrocarbons (HC) to furnish all types of oil S-organic compounds and hydrocarbons up to asphalt components. The increase of viscosity, density and content of heavy oil fractions with the grow of sulfur concentration as well as the increase of relative amount of S-organic compounds in the series mercaptanes → sulfides → thiophenes is in a good agreement with the regularities of the system HC-S0 evolution.6 It has also been suggested that the role of sulfur in the formation of oil components and sulfide ores makes these processes to some degree interconnected. This assumption is confirmed by the fact of joint deposits of metal sulfides and oil hydrocarbons.
Sulfides of different metals (Fe, Ni, etc) are also capable of formation of higher molecular hydrocarbons from CH4. Ethylene and propylene are generated without addition of elemental sulfur.9 Obviously, this fact is caused by higher mobility of sulfur in metal sulfides. In geochemical systems, the fugitivity of sulfur is very high.10 As a rule, the real surface of metal sulfides is depleted with metals. In this metal-deficient layer, sulfur can be presented in mono-,di- and polysulfide forms, i.e. in the state close to elemental.11,12 That is why metal sulfides are likely to be catalysts of hydrocarbons condensation.
Additionally, it is known that the contact of butane, butylene and other light HC with metal sulfides gives S-organic compounds contained in oils. Such reactions proceed through the interaction of HC with surface sulfur. The reactions rate increases with enhance of surface sulfur mobility.13 Thus, metal sulfides promote the formation of carbohydrate system containing sulfur.
The hypothesis proposed by the authors could help to investigate the correlations of sulfur concentration in oil with such characteristics of oil deposits as size, vertical zoning of different oils distribution as well as the dependence of oil properties on the prevalence of one or other metal (V, Ni).
The correlation of sulfur content in oil with the scale of its accumulation
It is obvious that the higher content of S0 in deep fluid the higher scales of condensation transformations of hydrocarbon gas to deliver more sulfur-bearing hydrocarbon system. Correspondingly, the contribution of a gas to oil and gas system will be lower. Indeed, such a regularity has been found14 both for some countries and continents Figure 1 and for a specific region (Western Siberia). Another evidence in favor of the hypothesis on the effects of sulfur concentrations on deposits and qualitative composition of naphthides is the existence of highly sulfuric oils and bitumen which deposits are 3-4 times bigger than other ones.15,16 This observed regularity is hardly correlated with the organic theory. According to the latter, sulfurization is, first of all, a consequence of a secondary process (sulfate-reduction). To follow this logic, one should expect that in the case of strong accumulation of sediments and increase of OM, the latter would be isolated from sulfate-containing waters, which access to OM might be hindered or even stopped. Therefore, an adverse regularity (as compared to those observed on Figure 1) would take place. Besides, one should bear in mind that the appearance of S in hydrosphere (in the form of easily soluble sulfates or sulfuric acid) occurs due to oxidative transformation of sulfides of rocks, i.e. it is more late process relative to the interactions of endogenic sulfur with ore and hydrocarbon components of the deep fluids. Sulfur, contained in the bio-systems, in the case of oil formation from only this source, could not provide the observed level of sulfur content in the majority of oils. Probably, it is just deep fluid that acts as a source of significant part of sulfur in oils. It is likely that low sulfur content can be attributed to the increased contribution of biogenic component to oil system.
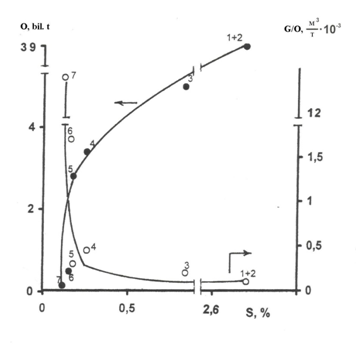
Figure 1 The influence of S concentration in oils on their resources (O) and the ratio of oils and gas resources (G/O). 1 -Saudi Arabia, 2 -Kuwait, 3 -Libya, 4 -Nigeria, 5- Indonesia, 6 -Australia, 7 -New Zealand.
The decrease of a part of gaseous component in oil and gas system Figure 1 caused by the increase of condensation effect of sulfur, should manifest itself in the characteristics of gases dissolved in oils. Indeed, statistical study of the data related to all oils of Russia have revealed14,15 that gas factor of some oils as well as the G/O value Figure 1 decreases with the growth of sulfur concentration Figure 2.
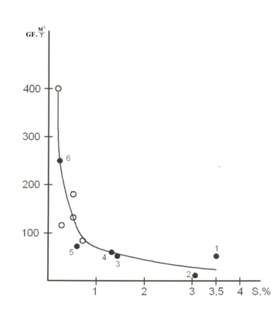
Figure 2 The Dependence of gas factor (GF) of oils on the content of S. O-oils of Bazhenov’s set of Salym field. 1-Romashkinskoye, 2-Arlanskoye, 3 - Tuimazinskoye, 4 -Mukhanovskoye, 5-Samotlorskoye, 6 -Zhirnovskoye (fields).
It has been suggested6 that the formation of centers (poles) of oil accumulation and transport of oil towards the surface of the Earth that is in a good accordance with the observed concentric zoning of the indices of oils composition of the basic complexes of Western Siberia.14 Two groups of indices are changed in different directions from a center to periphery. The content of sulfur and asphalt components, oil density, S/N ratio, content of CH4 relative to its homologs, total amount of trace elements, V content and VO/Ni-porphyrins ratio are reduced. At the same time, gas saturation and ratios of pristan / phytane, m-/o-xylene, Σ xylol/ethylbenzene, six- and five-membered napthenes, as well as the content of such metals as Ni, F, Mn, Cu and Cr are increased. It is likely that the regularity observed is conditioned by the increase of biogenic component from center to periphery.
Vertical zoning of bedding of the different composition oils
The methods of statistical and cluster analysis were used for the great number of oil basins.17 The results clearly show that low-sulfur and low-rubber oils are located in large depths. It has also been found that the closer to the surface of the Earth the higher concentration of S, aromatic, high-molecular compounds and asphalt elements. This regularity has not found any explanations from the viewpoint of traditional concepts. However, it can be rationalized from the position of the hypothesis proposed by the authors as follows. The ratio H2S and S0, which are contained in the deep fluid, should be changed as the fluid becomes closer to the surface of the Earth and oxidative processes are intensified. The content of S0 must be increased due to oxidation of H2S. The latter does not practically show any ability to enlarge hydrocarbon structures.7,8 Thus, the condensation processes leading to the formation of higher-molecular components should be strengthened as the fluid becomes closer to the surface of the Earth.
These ideas are confirmed by the fact that H2S has been found in light, deeply located oils having low content of S-organic compounds.18 In heavy oils, H2S is absent.19 High concentration of H2S is also observed in gas deposits and gas condensates in large depths. So, for example, its content in Astrakhan gas condensate (3-4 km depth) exceeds 24-25%.1,20 The possible explanation of vertical spatial alteration of oil composition from the viewpoint of the theory of organic origin due to the destruction of oil structures contradicts to the data21 on the increase of content of less stable sulfur-containing components with growth of depth.
The effect of relative content of V and Ni in oils on their characteristics
The presence of V and Ni, possessing reactivity and first of all catalytic properties, in the deep fluids22,23 can effect dramatically on the evolution of hydrocarbon fluid. This fact also relates to the introduction of metals (in the form of different compounds) into oil. The differences in the character of catalytic action of V and Ni and quantitative ratio of these metals in various fluid systems should impart the definite specificity to oils. The available data confirm this assumption. “Nickel” oils (Ni > V) are light, they are located in large depths, contains H2S and have low concentration of organosulfur compounds, rubbers and asphaltenes. “Vanadium” oils (V > Ni) are deposited in small or medium depths (1-3 km), they are heavy and have high content of sulfur and rubbers.24,25 The regularities observed correspond to the character of expected impact of these metals. Ni and V are clear antagonists with respect to hydrocarbon systems. Nickel belongs to hydrogenating agents and, therefore, can reduce S0 to H2S. Being active component of the catalysts of oil hydrodesulfurization, nickel facilitates the C-S bond cleavage in organosulfur compounds to afford H2S. Therefore, the predominance of Ni leads to weakening the condensation effect of S-containing component of the fluids. As a result, the condensation degree of oil and its sulfur content decreases. Contrary to this, V and its compounds, being catalysts of oxidation, in the presence of such oxidant as S0 should enhance the processes of oxidative dehydrocondensation of hydrocarbons. Of special importance is the ability of vanadium sulfide to oxidize H2S to S026 that, in its turn, enhances the condensation ability of S-containing component of the fluids and promotes to weighting of oil and growth of sulfur content in oils.
Para genesis of V + S with their increased concentrations is a characteristic feature of many hydrocarbon systems (heavy sulfur oils, bitumens, asphaltites, shales). In hard bitumens, the V content reaches 4.5, and in heavy sulfuric oils –6 kg/t.16,27 In deposits of asphaltites connected with sulfuric oil, the concentration of V is up to 6% with the formation of VS2.28
The comparison of properties of “vanadium” oils and shales represents a special interest for the study of V and S para genesis reasons. Geochemically, they correspond to each other with respect to V content (some kg per ton), conditions of depositing and carbon isotopes. This fact points to the connection of V concentrations with hydrocarbon generation in magmatic centers and is indicative of similar mechanisms of black shales formation with genesis of “vanadium’ oils.16 It is impossible to imagine that V and S could be obtained in such amounts from organic matter. This confirms the assumption that they are introduced by hydrocarbon fluids from the depths. Since the shales formation is accompanied by wide intake of H2S from the depth,16 one can suppose that it is just the ability of vanadium sulfide to generate S026 that intensifies the condensation processes of hydrocarbon systems.
The fact that “vanadium” oils are located closer to the surface of the Earth as compared to “nickel” oils is probably connected with high migration activity of V, joint movement of V and HC in the fluid flow, high affinity of vanadium to HC and its concentration in the course of hydrocarbons evolution. The content of V in oil shale is two times higher than in sediments, and the generation of its sulfide deposit is possible only in the case of extremely high-sulfur oils.16 In the US, 2/3 of vanadium is extracted from oil.28 Unlike this, nickel sulfide is crystallized in rocks as huge massifs.
In the course of the fluid movement towards the surface of the Earth and intensification of oxidizing conditions, the content of the oxidized form (V5+), which shows migration activity,29 can increase. The investigations of rock cores of Western Siberian oil beddings using a method of secondary ionic mass spectrometry30 has shown that intensity of V peaks increases with decrease of deposits depth. As far as Ni is concerned, such regularity is not observed.
Isotopy of Oils Carbon
To prove the possibility of oil abiogenic synthesis in depths of the Earth one should use isotope ratios of carbon in various hydrocarbon systems. It is known that general trend of carbon oxidation is accompanied by the following scheme of 13С isotope enrichment: СН4 → С → СО1-3 → СО2 → СО3-2.31 The concentration of heavy isotope (δ13С) for СН4 (biogenic and geothermal) is from -30 to -70, СО2 atmospheric -7, and marble -3.0 ‰ (Faure, 1986). From the regularity of carbon isotope composition alteration in the course oxidability (reducibility) changing follows that in the case of multicomponent carbon-containing systems, where quantitative ratio of components with various degree of oxidability (reducibility) varies, the range of δ13С changing can reach high values. So, for proteins, carbohydrates, cellulose, lignin and lipids31] these values are -17...-28 ‰, while for plants, OM of modern sediments and oil they equal -6...-34,-10...-32,-18...-34, respectively (Faure, 1986). In kerogen of metamorphized rocks, due to the effect of oxidation, reduction, etc. the spread in δ13С values is from -33 to -13; in various components of OM -from -36 to -16.5; in bitumens – from -36 to -15.5 ‰.32 Therefore, the “direct” application of characteristics of carbon isotope composition of complex hydrocarbon systems for the research of oil genesis is not quite fruitful. More reasonable is the usage of isotope ratios for narrower fractions of hydrocarbon systems and individual HC.
According to the work,33 δ13С value for СH4 of different fields is -36… -46 ‰. For liquid alkanes of oil these values are changed to -31...-38 ‰, and for aromatic fraction – to-27...-30 ‰.34 The enrichment with 13С isotope is observed as follows35 СН4 (-38...-46) < liquid HC of oil (-27...-38) < oil bitumens, asphaltites (-18...-24) < mantle СО2 (-7.2) < heavy diamonds (-2.0...-7.2 ‰). As a whole, carbon in natural gas is depleted with 13С more strongly than the associated oil.36 It is known37 that asphaltenes of oils in comparison with other oil components have the heaviest carbon isotope composition. The growth of asphaltenes content in oils of Western Siberia leads to the increase of 13С concentration in both asphaltenes and oils.14 The characteristics of bitumens inclusions to minerals, formed in hydrothermal processes, also confirm the growth of heavy carbon isotope composition during the increase of condensation degree.38 For liquid oil-like bitumens, malthas, asphaltenes and kerites the values of δ13С are -23...-33; -24; -23; and -19 ‰, respectively. Similar regularity is observed for individual HC. The enrichment with heavy isotope occurs with the increase in molecular weight of gas systems C1-C4.36 In particular, in case of giant fields of China the value δ13С is -34 for СН4, -26 for С2, -25 for С3 and - 23 ‰ for С4. In hydrothermal field of Juan-de-Fuca ridge, the δ13С value of CН4 is -51...-54, for С2-7 (basically, alkanes) the enrichment of 13С reaches-20...-25, and for toluene-20 ‰.25
Rudenko and Kulakova35 have proposed to consider the abiogenic polycondensation of СН4 as a model of isotope fractionating in a course of HC transformations. Catalytic polycondensation of СН4 induces the following enrichment of 13С: СН4 → volatile products of polycondensation → nonvolatile products.39 The direction of isotopes ratio changing, observed for the components of real oils and naphthides (see above) is similar to those shown in the above-mentioned model. Therefore, the conclusion that the carbon isotope composition in oil systems is determined by isotope fractionating in condensation abiogenic processes and testifies to their essential contribution to oil formation, is quite justified.
Following the logic of the concept of oil origin only from OM of sedimentary rocks, one should expect that oil would be enriched by heavy isotope in comparison with OM, since the bonds, formed by an easy isotope, are less strong,36 and the destructive transformations of OM to oil should involve a removal of the structures containing an easy isotope. However, it is difficult to find the expected enrichment (see above).
It has been reported31,35 that the isotope composition of deuterium in oils and СН4 of oil and gas deposits also speaks in favor of a deep origin of these hydrocarbon systems. It is necessary to remind that from 450 discovered oil fields only 54 are connected with sedimentary rocks,40 while according to the data41 from 600 sedimentary reservoirs in the world only 1/3 is oil-bearing.
Isopoty of oil sulfur
The result of comparison of S isotope composition of mantle origin, marine (oceanic), sediment, bio-systems as well as oil itself might be the major argument in favor of one or another opinion about S source in oils. The value δ34С in oils changes from -8 to +32 ‰, but for a significant number of fields (marine origin, cretaceous, tertiary and late triassic age, Western Siberia) this range is significantly less (from-7 to +5 ‰).14,36 Various contribution of mantle, sulfate S and S of bio-systems can be one of the reasons of variations of δ34С values.
Essential difference of isotope compositions of S in mantle from meteoric is improbable. In this connection, it is necessary to pay attention to close δ34С values of brimstone S (+2.5... +4.0 ‰) of salt domes containing oil (Mexico) and mantle S.36 Even in a case of only mantle origin of S oils, some changing of its isotope composition is possible due to the variation of isotope composition of mantle S. The latter can depend in a certain degree on temperature, magma structure, S fugitivity. So, for sulphide magmatic minerals the δ34С deviations are -11... +9 ‰.36
Considering the possibility of occurrence of sulphatic S in oil, one should bear in mind that H2S evolved during the reduction possesses weaker ability (in comparison with S0) to “sulfurize” hydrocarbon structures. Besides, bacterial sulfate-reduction (at diagenesis stage, low temperatures) leads to strong depletion of the reduced forms of sulfur with heavy isotope. The values δ34С of sulphate S in hydrosphere and sulphates of sedimentary rocks are +20 … +30 ‰. In H2S, the value δ34С reaches -11...-50 ‰, and in a sulphide phase in diagenesis zone this value is -10...-40 ‰36,42 that strongly differs from isotope composition of oil S. It is unlikely that “sulfurization” of hydrocarbon structures proceeds intensively during low-temperature sulfate-reduction. Under thermal sulfate-reduction the rate of this process can increase. At the same time the growth of temperature should lead to fading the effect of isotope fractioning. In some cases S in H2S and initial sulphates under the conditions of high-temperature sulfate-reduction is not distinguishable on isotope composition, and the value δ34С in H2S gases of large hydrocarbon provinces is +10 … +15 ‰.42 Therefore thermal sulfate-reduction can promote to enrichment of oil sulfur with heavy isotope. Certain enrichment can occur also because of the introduction of bio-structures in oil, where, as it is known36 the value δ34С is +15... +20 ‰. In this connection, the particular interest for revealing the sources of oil S represents isotope composition of oil S of Western Siberia, where the reservoir waters contain no sulfates, and the value δ34С for these oils is -7.4... +4.3 ‰ .14 If to admit that the absence of sulfates is caused by their total reduction with "incorporation" of S into oil, one should expect high enrichment with heavy isotope (up to +20... +30 ‰). One can conclude here that not sulfate-reduction, but S of bio-systems and mantle S should be the source of S in these oils. However bio systems, owing to low concentration of S, cannot provide the sulfur content observed in the majority of oils.43 The decrease of sulfur content and possibly connected with it the increase of the contribution of bio systems may lead to some enrichment of oil with S heavy isotope that is really observed for this region Figure 3 and for other oils and condensates.14
Thus, the interaction of endogenic CН4 (its closest homolgs) and S0 followed by the condensation processes and the formation of oil components does not contradict a complex of the data on characteristics of real oil systems. The data on isotope composition of C and S of oils are in agreement with regularities of isotope fractioning of C and S, occurring during the evolution of natural systems .44
The data on composition of endogenic gas fluids and reactivity of their components (CH4, H2S, S0, metals, etc.) allows one to rationalize the possibility of realization of one of the routes to the formation of abiogenic part of oil as well as to explain the regularities in variations of real oil systems indices.
Elemental sulfur is able to initiate the oxidative dehydrocondensation transformations of hydrocarbons including methane and its close homologs. The influence of S0 on CH4 can lead to the generation of all types of HC and S-organic components of oils. The higher concentration of sulfur in the fluid the more intensive the processes of formation of oil components and the more sulfur system will be reached. At the same time, the part of non-condensed gas in the system should decrease. The available data on several oil fields support the point of view presented in the work.
The increase in sulfur content, viscosity and density of oil with the decrease of occurrence depth observed in the majority of oil fields can be explained by intensification of oxidative conditions and oxidation of H2S to S0. H2S is not able to initiate the condensation transformations of HC, therefore the deeper-embedded hydrocarbon systems are less condensed and contain significant amounts of H2S (light oils, gas condensates, gases).
Metals V and Ni due to the peculiarities of their catalytic properties should exert adverse action on the hydrocarbon systems. Vanadium, being the carrier of oxidative action, should strengthen oxidative dehydroconsensation transformations of HC. Besides, vanadium sulfide occurring in hydrocarbon systems can oxidize H2S to S0 and additionally enhance the condensation transformations. Contrary to this, Ni is hydrogenating agent and it is capable to increase H2S content as well as to destroy the C-S bond to afford H2S. These features of Ni should promote to the formation of more light and less sulfur oils.
The compositions of real oils with predominance of V or Ni are in good agreement with the assumed character of influence of these metals on hydrocarbon systems. The data on isotope ratio of C and S in oils, their fractions, individual compounds do not contradict to the notions on participation of endogenic CH4 and S in the formation of abiogenic part of oil and gas systems.
None.
The author declares no conflict of interest.

©2017 Lurie, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.