International Journal of
eISSN: 2475-5559


Research Article Volume 1 Issue 2
1State Key Laboratory of Chemical Engineering, Tsinghua University, China
2State Key Laboratory of Organic-Inorganic Composites, Beijing University of Chemical Technology, China
Correspondence: Jian Chen, State Key Laboratory of Chemical Engineering, Tsinghua University, China
Received: September 09, 2016 | Published: October 7, 2016
Citation: Chen J, Zhang R, Yu Y, et al. Measurement of CO2 solubility in aqueous branched polyamine solutions. Int J Petrochem Sci Eng. 2016;1(2):31-34. DOI: 10.15406/ipcse.2016.01.00008
In energy industry as petrochemicals and electricity generation, absorption technology is looked as the main one for CO2 capture from flue gases. In this work, absorption solubility of CO2 in aqueous solutions of four branched polyamines were determined at 313.15 K and 393.15 K using the constant-volume method combined with gas chromatography analysis. The relationship between molecular structure of these polyamines and absorption performance is discussed. It is found that the capture performance is affected by the number of amino groups, the carbon number between the amino groups and the chain length of polyamines. The corresponding values of CO2 absorption heat were also estimated using the Gibbs-Helmholtz equation and were analyzed from the view of molecular conformation.
Keywords: CO2 capture, absorption, branched polyamine, solubility
Global climate warming associated with increased emissions of CO2 from anthropogenic sources have become one of the most critical worldwide issues of the current age. A multitude of technological advances1–3 have been developed to reduce CO2 emissions from combustion exhaust gases from petrochemicals and electricity generation. Nowadays amine-based aqueous solutions are the most commonly used absorbents for the absorption process.4 In this case, finding new solvents that offer highly cyclic absorption capacity is important. Compared with monoamine solvents, polyamines solvents 5 are expected to have lower volatility along with higher CO2 loading capacity and mass transfer rate. For example, diethylenetriamine (DETA) entails a higher mass transfer rate, 6 higher cyclic capacity,7–9 and significant lower heat of absorption 10 than monoethanolamine (MEA). Tetraethylenepentamine (TEPA) shows outstanding potential for CO2 absorption, and it can maintain a high absorption rate as well as cyclic absorption capacity.11 One mole TEPA removes three times more CO2 per cycle than 1.0 mole MEA. Cyclic absorption capacity is the solubility difference between absorption and desorption. These investigations reveal that polyamines make a good prospect for chemical absorption. Singh et al showed that an increase in chain length between the amine and different functional groups, result in a decrease of absorption rate whereas, the absorption capacity was increased in most absorbents.12,13 Machida et al.14 observed that alkyl chain length between two amines has an important role in CO2 solubility.14 Although some fragment solubility data for polyamines have been reported in previous literature, the data for the polyamines in different structures are insufficient.
In this work, the vapor-liquid phase equilibria of CO2 in aqueous solutions of four branched polyamines were measured at 313.15 K (absorption process) and 393.15 K (desorption process). Based on the solubility data, the corresponding absorption heats () were calculated to analyze the energy consumptions for CO2 capture with these solutions.
Materials
CO2 and N2 with a volume fraction of 0.99999 were supplied by Millennium City Gas. The solvents used are listed in Table 1. The solution was prepared using ultrapure water, which was taken from the Center 120 FV-S ultrapure water machine. The resistivity of ultrapure water is 18.2 MΩ·cm at 298.15 K. All components were used without further purification.
Component |
Abbreviation |
Molecular formula |
CAS number |
Purity (%) |
Source |
3,3’-Diamino-N-methyl- dipropylamine |
DAMDPA |
C7H19N3 |
105-83-9 |
>98 |
TCI Shanghai |
N,N Dimethyldipropylenetriamine |
DMDPTA |
C8H21N3 |
10563-29-8 |
99 |
Sigma-Aldrich |
N,N-Dimethyl-1,3 propanediamine |
DMPDA |
C5H14N2 |
109-55-7 |
99 |
Alfa Aesar |
N,N,N’,N’-Tetramethyl-trimethylenediamine |
TMTMDA |
C7H18N2 |
110-95-2 |
>98 |
TCI Shanghai |
Table 1 Chemicals used in this work
Apparatus and procedure
The constant-volume method, combined with gas chromatography analysis was used to measure the differing levels of solubility. A detailed description of the vapor–liquid equilibrium apparatus can be obtained from previous literature.15,16 It is comprised of two stainless steel tanks for buffer and reaction. Both tanks are equipped with temperature transducers (PT-100, Kunlunhaian Co.) and pressure transducers (JYB-KO-HAG, Kunlunhaian Co.). The accuracy of the temperature and pressure transducers is ±0.1 K and ±0.5%, respectively. In accordance with the Peng-Robinson (PR) equation, the precise amount of CO2 in the gas phase was determined using its volume, pressure, and temperature.17 The principle of this method is to accomplish a known volume of gas with the known-volume polyamine solutions. The amount nallCO2 of CO2 gas introduced into the reaction tank was determined by the change in the buffer tank, before and after injection. After equilibrium was achieved at a constant temperature, the amount of CO2 gas absorbed in the solutions nlCO2 was equal to nallCO2 subtracted by the amount of CO2 in the vapor phase ngCO2 . The CO2 solubility , αco2 , in the liquid phase was defined as nlCO2 divided by the mole number or mass of amine. The experimental error of CO2 loading is ±8%.
At low partial pressure (<10 kPa), the pressure transducer error may have a greater impact on results. Therefore, the CO2 partial pressure was obtained using gas chromatography (Agilent 7890). The gas phase is primarily composed of CO2, with minor components of N2, H2O, and amine. The partial pressure of amine is small enough to be considered negligible. The partial pressure of water was calculated according to Raoult’s law. Prior to the introduction of CO2 into the reaction tank, the partial pressure of N2 was calculated by subtracting H2O partial pressure from total pressure. Correspondent to the increase in CO2 amount, the solution’s volume change remained negligible and the N2 partial pressure was assumed constant. The apparatus was verified by determining the solution of CO2 in 30 mass% MEA solution at T=313.15K and 393.15K. The results are compared with reported data.18–20 Our results are agree with reported data.
CO2 solubility levels in aqueous solutions of four branched polyamines at 313.15 K and 393.15 K were measured using the constant-volume method combined with gas chromatography analysis, and the results are shown in (Figures 1–3). The concentration of amines were set to be 2.5M, which correspond to about 25% - 40% in weight percentage, which is the usual range used in CO2 absorption processes. The measured corresponding solubility levels are expressed in two forms, both involving the mole number of CO2; in one form, it is dissolved in per mole of amine, and in the second, per kilogram of amine. The relationship between solubility and molecular structure Table 2 can be also seen in Figures 1–3.
Abbreviation |
DMPDA |
TMTMDA |
DAMDPA |
DMDPTA |
Molecular Structure |
|
|
|
|
Table 2 Molecular Structure of Chemicals used in this work
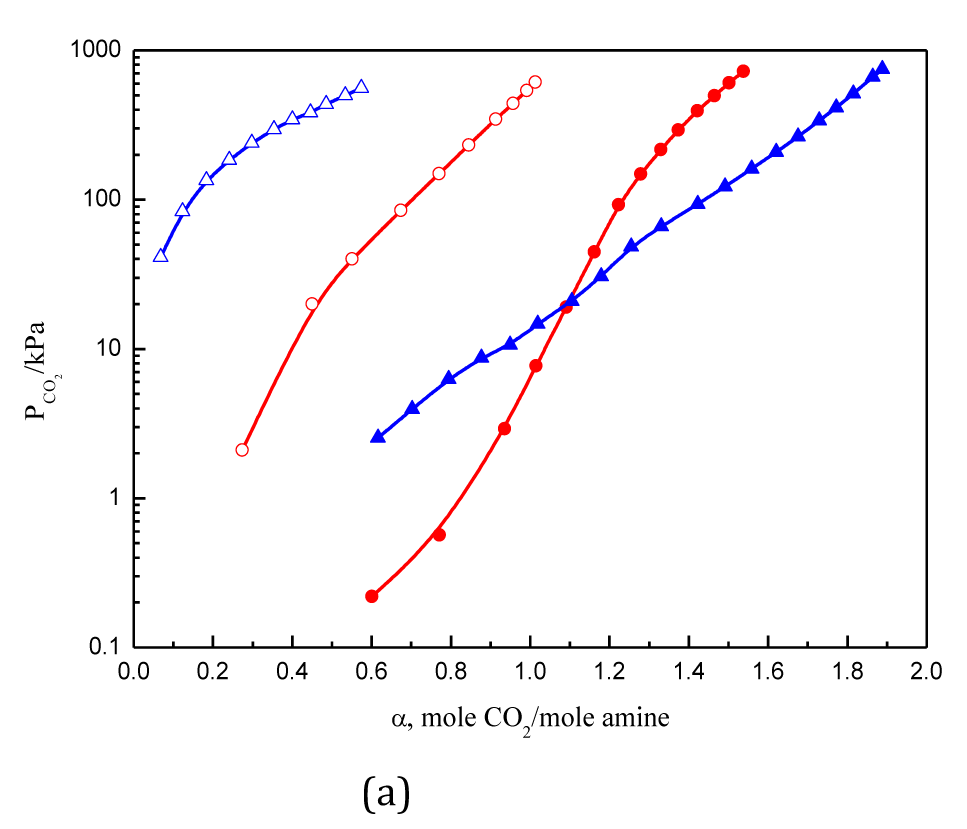
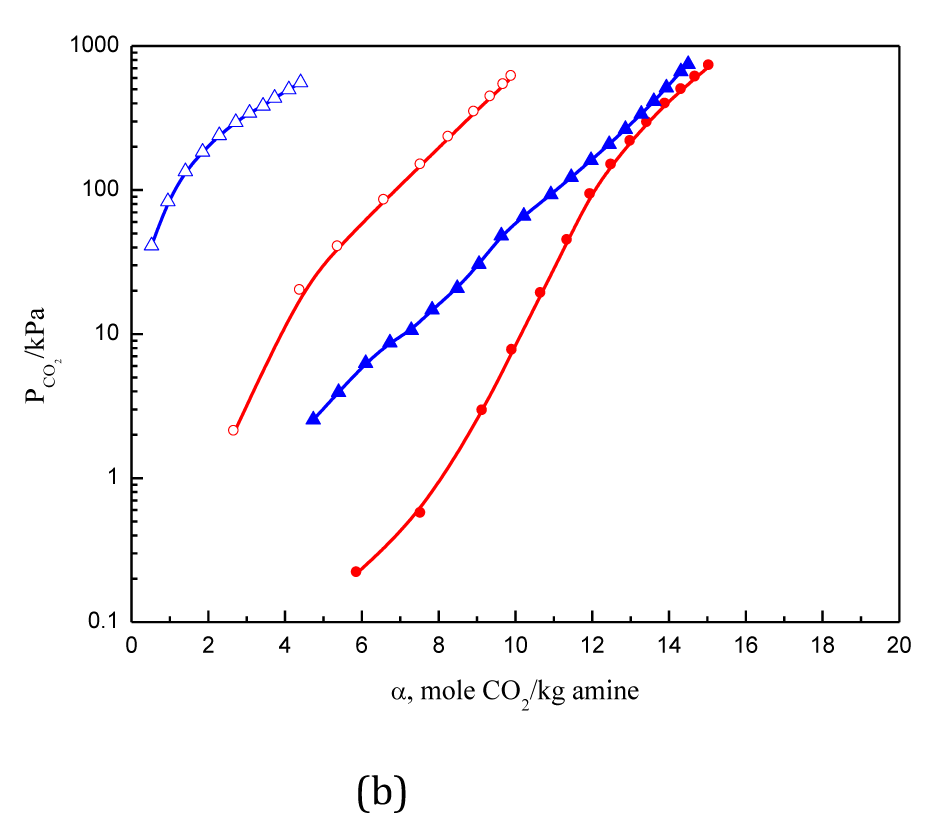
Figure 1 Solubility of CO2 in 2.5 mol·L-1DMPDA, and TMTMDA solutions.
●: DMPDA 313.15 K; ○: DMPDA 393.15 K; ▲TMTMDA 313.15 K; △TMTMDA 393.15 K.
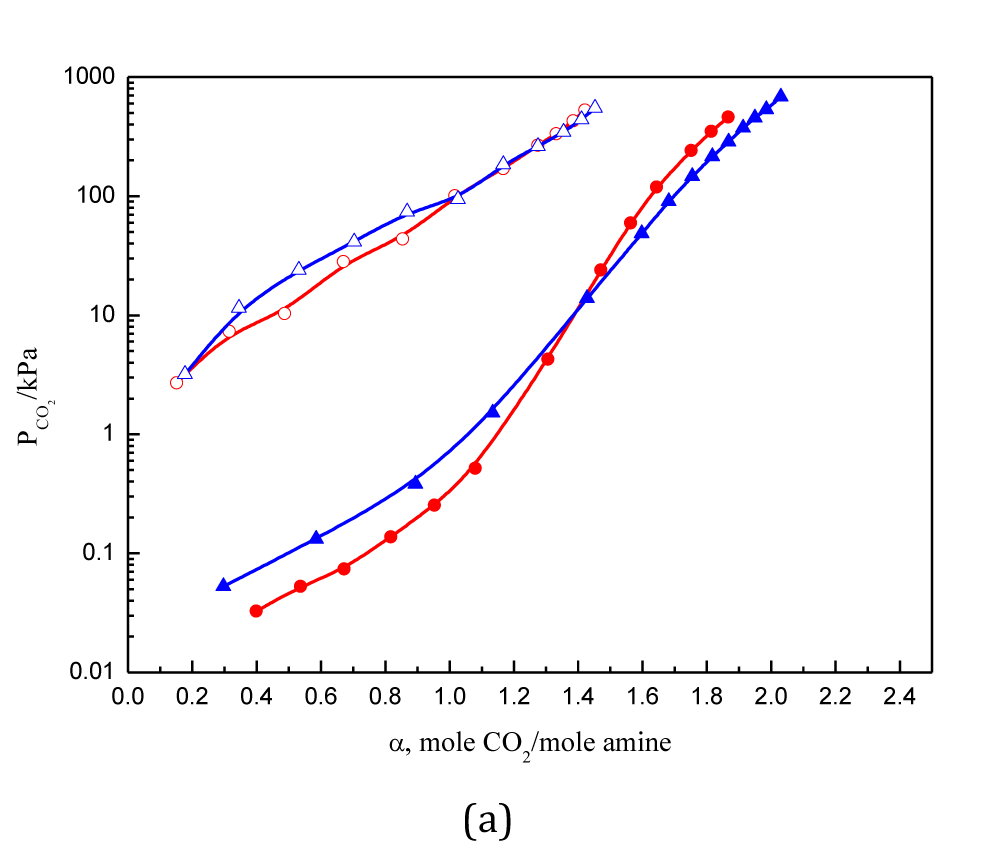

Figure 2 Solubility of CO2 in 2.5 mol·L-1 DAMDPA, and DMDPTA solutions.
● DAMDPA 313.15 K; ○ DAMDPA 393.15 K; ▲ DMDPTA 313.15 K; △ DMDPTA 393.15 K.


Figure 3 Solubility of CO2 in 2.5 mol·L-1 DMDPTA and DMPDA solutions.
■ DMDPTA 313.15 K; □ DMDPTA 393.15K; ● DMPDA 313.15K; ○ DMPDA 393.15K.
CO2 solubility in aqueous solutions of four branched polyamines are measured and compared with each other. The cyclic absorption capacity increases with an increase in the relative amount of tertiary amino groups in the amines, while primary and secondary amines have similar absorption capacity. For polyamine containing three or more amino groups, the situation is more complex due to impact of the steric hindrance. The high theoretical absorption capacity of tertiary amines, steric effects, and the instability of bicarbonates should be simultaneously accounted for the total results. As the number of amino groups increases, the ability to absorb CO2 declines. The value of absorption heat is crucial for the energy consumption to desorb CO2, and it decreases as alkyl groups on nitrogen atom increases.
This work was supported by National Science and Technology Support Program of China (No. 2015BAC04B01) and the National Natural Science Foundation of China 21276010 and 51373019).
The authors declare no conflict of interest.

©2016 Chen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.