International Journal of
eISSN: 2475-5559


Research Article Volume 4 Issue 4
1Chemistry Department, Faculty of Science, Ain shams University Cairo, Egypt
2Department of Polymers and Pigments, National Research Centre, Cairo, Egypt
Correspondence: Afify MF, Chemistry Department, Faculty of Science, Ain shams University Cairo, Egypt, Tel 01022232443
Received: September 06, 2019 | Published: September 30, 2019
Citation: El-Ghaffar AMA, Youssef EAM, Afify MF. High performance metal stearates thermal stabilizers for poly vinyl chloride. Int J Petrochem Sci Eng.2019;4(4):162-168. DOI: 10.15406/ipcse.2019.04.00116
Low thermal stability of poly vinyl chloride (PVC) is one of the most serious problems that facing its processing. In the present study some single and mixed metal stearate soaps were prepared, characterized and evaluated for their performance in enhancing and imparts the thermal stability of PVC. Accordingly, calcium stearate, barium stearate, zinc stearate and also the mixed metal stearate salt of calcium, zinc and barium were prepared by the double decomposition of sodium stearate and single or mixed metal acetate solutions. The prepared metal stearates were characterized via spectroscopic analysis (FT-IR and XRD) in addition to micro analysis and thermal gravimetric analysis (TGA). The results showed that the prepared metal stearates are good thermal stabilizers for PVC; also the thermal stability of the prepared (Ca/Ba/Zn) stearate was enhanced when melamine, ammonium poly phosphate, magnesium hydroxide and Ca/Zn phosphate are mixed with it.
Keywords: poly vinyl chloride, thermal stabilizer, metal stearates
PVC, Poly vinyl chloride; XRD, X Ray Diffraction; TGA, Thermo Gravimetric Analysis; FT IR, Fourier Transform Infra Red; Ca/Ba/Zn, Calcium, barium, zinc; Ba/Zn Barium, zinc, Ca/Zn, Calcium, zinc; DOP, Dioctyl phathalate; ESBO, Epoxidized soya bean oil; min, Minute
PVC is an important thermoplastic material on account of its versatility and low cost, however one major drawback of PVC is that it decomposes at temperature lower than its processing temperature, thermal degradation of PVC is the result of dehydrochlorination on exposure to heat1,2 which generates polyene sequences in polymer chains that may produce an undesirable color in material during its molding and use,3,4 in the advanced stages of degradation PVC losses mechanical, electrical and rheological properties as a result of chain scission and cross linking.5 During PVC processing, dehydrochlorination of PVC affects on mass loss and as a result of that color change to yellow, orange, red, brown and finally black due to the number of conjugated bonds that formed.6 To overcome this problem, thermal stabilizers used to protect PVC from chemical degradation effects of heat, without the use of thermal stabilizers PVC could not be the widely used polymer.7 During the polymerization process the normal head to tail free radical reaction of vinyl chloride deviates from the normal path and result in sites of lower chemical stability or defect sites along some of the polymer chains, thermal stabilizer technology depends on prevention or repairing these defect sites.7 Thermal stabilizers include a wide variety of metallic soaps, commercial heat stabilizers in the market are actually synergetic mixtures of various compounds (mixed metal stearates and metal hydroxides),6 Magnesium hydroxide can be used with metallic soaps to enhance the thermal stability of PVC because it release weak Lewis acid (magnesium chloride) when it react with hydrochloric acid.
In general, the stabilizers are classified as lead salts,8 organic tin,9 soap salts10 and rare earth stabilizers.11 Stabilizers play the role in stability mainly through absorption of hydrochloric acid (HCl) released from partial PVC decomposition or substitution of labile sites of partially decomposed PVC macromolecules.5 Application of lead salts and organic tin stabilizers are limited due to their toxicity, even they have high efficiency to stabilize PVC. The long-chain Carboxylate of metal ions are compounds of considerable commercial importance and are employed in various fields. For example, zinc soap is used as an ant moisture agent, a lubricant in polymers,12 or a dispersing agent in pigments. Mixed metal stearates predominate in the flexible PVC applications in the United States and they find competition from lead based products in Europe for electrical wires and cable coatings.7 In Europe mixed metal stabilizers are preferred for extruded rigid building profiles because they provide good weathering and physical properties to PVC in this use, The most popular commercial products are combination including Ca/Zn, Ba/Ca/Zn and Ba/Zn, Calcium/zinc mixtures are used to stabilize PVC food packing, mineral water bottles and pharmaceutical containers thought the world7 Calcium/zinc has synergistic effect and present favorable stabilization; however Ca/Zn stabilizers have some disadvantages in long term stability due to its zinc content.
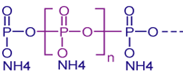
Hence extra substance must be added so as to improve the efficiency of stabilization such as epoxidized soya bean oil13 Melamine which is an organic base which contains 66% nitrogen by mass so it can capture hydrochloric acid and, if mixed with resins, has fire retardant properties due to its release of nitrogen gas when burned or charred,14 Ammonium polyphosphate which is an inorganic salt of polyphosphoric acid and ammonia containing both chains and possibly branching. Its chemical formula is [NH4 PO3] n and it can react with hydrochloric acid; ammonium polyphosphates decompose to form ammonia and phosphoric acid so it act as fire retardant.15 Devrim Balkose et al.,16 studied the synergism of calcium and zinc soaps on thermal stabilization of PVC using solvent casted films of PVC having commercial zinc and calcium soap in different proportions. The metal soaps were characterized by X-ray diffraction and X-ray emission techniques, The films that were heated at 80°C for 4 hr for the removal of the last traces of solvent methyl ethyl ketone were tested for thermal stability by heating at 160°C for 30 min. heated films were characterized by infrared and ultraviolet-visible spectroscopy, DSC and TGA methods the migration of zinc, calcium and hydrogen cations to water at 80°C was also studied. UV spectroscopic analysis and ion migration indicated synergism on PVC heat stability for 4:1 Ca to Zinc soaps ratio
Double decomposition (precipitation) process was used for preparation of single and mixed metal stearate soaps.17,18
Preparation of calcium, barium and zinc stearates
These salts were prepared by two steps:
Preparation of mixed Ca/Ba/Zn-stearate
Sodium stearate was prepared as mentioned above, and then Ca/Ba/Zn-stearate was prepared by addition of sodium stearate solution gradually to aqueous solution of Ba, Ca, and Zn-acetate mixture, the prepared Ca/Ba/Zn-stearate was purified as mentioned before
Commercial stabilizer (Akropn 2611 px), prepared single and mixed metal stabilizers were added in different ratios to PVC compound formulation that contain the following recipe.
Thermal stabilizer |
ESBO |
DOP |
Calcium carbonate |
PVC K70 |
3 or 5 |
2 |
50 |
60 |
100 |
Other additives such as Magnesium hydroxide, melamine, ammonium poly phosphate and Ca/Zn phosphate were added with the prepared metal stearates in order to increase the stabilization efficiency. All formulations were mixed on Bara bender at 150°C and 80 r.p.m., and then were prepared on a two roll mill of diameter 470 mm and width 300 mm with speed of slow roll at 24 rev./min. A sheet of 1 cm width and 20 cm length was prepared and tested in Metrastat static heat stability oven model IR-700 at 200°C for 2 hours in normal air according to ASTM D-2115.
Micro analysis of carbon and hydrogen
Micro analysis of carbon and hydrogen for prepared metal stearates were carried out on vario EI Elementar apparatus (Germany). The results are illustrated in Table 1. It is quite clear that the data of micro analysis given agree to a large extent with the calculated values for the proposed structures.
Molecular formula |
% Hydrogen |
% Carbon |
|
|
|---|---|---|---|---|
Theoretical |
Found |
Theoretical |
Found |
|
(C18H35O2)2Ca.2H2O |
11.6 |
10.3 |
67.25 |
66.7 |
(C18H35O2)2Ba.2H2O |
10.09 |
9 |
58.48 |
58.6 |
(C18H35O2)2Zn.2H2O |
11.16 |
8.8 |
64.7 |
64.5 |
(C18H35O2)2Ca/Ba/Zn |
11.86 |
9.6 |
72.7 |
75.7 |
Table 1 Micro analysis of carbon and hydrogen.
Determination of metal content by loss in ignition method
The metal stearate salts were ignited at 1000°C for 1 hr in a muffle until the all organic content were lost then the percent of metal was calculated from the remained metal oxide (Table 2).
Molecular formula |
% Metal |
|
|---|---|---|
Found |
Theoretical |
|
(C18H35O2)2Ca.2H2O |
6.51 |
6.59 |
(C18H35O2)2Ba.2H2O |
20.21 |
19.5 |
(C18H35O2)2Zn.2H2O |
12.36 |
10.34 |
(C18H35O2)2Ca/Ba/Zn |
11.856 |
12.9 |
Table 2 Metal content by loss in ignition method.
IR spectra of the prepared metal stearates were obtained at a resolution of 4 cm−1, between 4000 and 400 cm−1, using a model FT-IR 6100. The IR spectra of the prepared Ca-stearate, Ba-stearate, Zn-stearate and Ca/Ba/Zn-stearate give a good confirmation of their structures as shown in Figure 1A-1D. The absorption bands at 3428, 3426, 3386 and 3430 cm-1 can be attributed to water bonded to crystal respectively, while bands at 2918, 2917, 2919 and 2918 cm-1 can be attributed to the bonded methyl group stretching, absorption bands at 2850, 2849, 2851 and 2850 cm-1 can be attributed to the bonded Methylene group stretching, the bonded carboxylic group anti symmetric vibration bands appeared at 1578, 1512, 1545 and 1574 cm-1, while the bonded CH bending bands appeared at 1471, 1444, 14668 and 1468 cm-1, absorption bands that appeared at 1112, 1110, 1045 and 1110 cm-1 can be attributed to the bonded C-O stretching of Carboxylate group, finally bands that appeared at 724, 717, 709 and 721 cm-1 can be attributed to the Carboxylate bending
Thermal gravimetric analysis (TGA)
The thermal stability of the prepared metal stearates was investigated using TGA analysis under nitrogen gas at a heating rate of 10oC/min over a temperature range from room temperature up to 1000oC using TGA {Perkin-Elmer 7 series USA},Thermal gravimetric results are illustrated in Figures 2A-2D respectively. From TGA results of the prepared metal stearates we can conclude that in the first stages the salts are nearly stable while weight loss was 19.34% at 300oC for calcium stearate, 7.67% at 315oC for barium stearate, 13.1% at 170oC for zinc stearate and 12.239 % at 310oC for Ca/Ba/Zn stearate and this may be attributed to loss of water molecules from prepared salts. Above 300oC a sharp loss due to stearate degradation was observed for all prepared metal stearates except zinc stearate where its sharp loss observed at 170oC and this mean that zinc stearate is lower stable than other salts. All organic content was lost at 500 C for all prepared metal stearates and the remained weight was a metal oxide
X-ray diffraction (XRD) analysis
The results of XRD for the prepared (Ca, Ba, Zn and Ca/Ba/Zn-stearates) are illustrated in Figure 3A-3D respectively and Table 3.
XRD of Ba/Ca/Zn-stearate |
Standard ICDD of Ca- stearate card No. 25-1570 |
XRD of prepared Ca-stearate |
Standard ICDD of Ba-stearate card No. 09-0848 |
XRD of prepared Ba-stearate |
Standard ICDD of Zn-stearate card No. 05-0079 |
XRD of prepared Zn-stearate |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Relative intensity |
d(A0) |
Relative intensity |
d(A0) |
Relative intensity |
d(A0) |
Relative intensity |
d(A0) |
Relative intensity |
d(A0) |
Relative intensity |
d(A0) |
Relative intensity |
d(A0) |
I/I0 |
I/I0 |
I/I0 |
I/I0 |
I/I0a |
I/I0 |
I/I0 |
|||||||
- |
- |
100 |
30.9 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
86.4 |
21.364 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
74.1 |
15.12 |
5 |
15.4 |
100 |
15.84 |
28 |
15.9 |
100 |
15.34 |
- |
- |
- |
- |
100 |
14.57 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
31.3 |
10.99 |
5 |
10.8 |
- |
- |
- |
- |
- |
- |
13 |
10.5 |
19.8 |
10 |
24.5 |
9.37 |
10 |
24.8 |
50.29 |
24.12 |
13 |
9.57 |
25.68 |
9.15 |
- |
- |
- |
- |
46.3 |
8.84 |
- |
- |
- |
- |
- |
- |
- |
- |
27 |
8.34 |
33 |
8.046 |
20.9 |
7.41 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
48.1 |
4.505 |
40 |
4.41 |
44.73 |
4.37 |
17 |
4.58 |
31.57 |
4.58 |
100 |
4.53 |
36.4 |
4.507 |
47.8 |
4.29 |
25 |
4.3 |
22.11 |
4.104 |
- |
- |
- |
- |
- |
- |
- |
- |
62 |
4.17 |
- |
- |
- |
- |
7 |
4.18 |
- |
- |
- |
- |
- |
- |
46.8 |
4.027 |
- |
- |
- |
- |
7 |
4.02 |
5.73 |
4.09 |
- |
- |
- |
- |
41.6 |
3.846 |
- |
- |
- |
- |
8 |
3.87 |
10.86 |
3.91 |
67 |
3.92 |
46.9 |
3.88 |
58.5 |
3.707 |
- |
- |
- |
- |
7 |
3.68 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
20.78 |
9.47 |
12 |
11.8 |
21.13 |
11.46 |
100 |
13.28 |
100 |
13.8 |
- |
- |
- |
- |
26.77 |
5.821 |
5 |
4.46 |
5.3 |
4.41 |
3 |
6.36 |
1.6 |
6.435 |
- |
- |
- |
- |
12.35 |
3.87 |
- |
- |
- |
- |
7 |
2.26 |
7.1 |
2.37 |
Table 3 D values dA0 and relative intensity I/I0 of X-ray diffraction patterns of prepared stearates
There is some agreement in the position and intensity of some of the principle phases of the prepared Ca, Ba, Zn and Ca/Ba/Zn-stearates with that of standard ICDD, but also some variation in other minor peak which may be due to other constituents of traces element's present.
Evaluation of the prepared metal stearates as thermal stabilizers for PVC
The prepared metal stearates were evaluated as thermal stabilizers for PVC by measuring Static thermal stability for the prepared formulations in metra stat oven model IR-700 at 200oC for 2 hr in normal air , the results are illustrated at Tables 4 & 5 and Figure 4A & 4B Poly (vinyl chloride) compositions degrade by discoloration on prolonged exposure to heat.
Thermal stabilizer |
Stability time (minute) |
akropan 2611 px (3phr) |
40 |
Ca stearate (3phr) |
15 |
Ba stearate (3phr) |
20 |
Zn stearate (3phr) |
0 |
Ca/Ba/Zn stearate (3phr) |
52 |
Table 4 Stability time of prepared stabilizers against commercial one akropan 2611 px 3phr
Thermal stabilizer |
stability time (minute) |
akropan 2611 px (5 phr) |
50 |
Ca/Ba/Zn stearate (5phr) |
58 |
Ca/Ba/Zn stearate + Mg(OH)2 (4:1)phr |
48 |
Ca/Ba/Zn stearate + ammonium poly phosphate (4:1) phr |
42 |
Ca/Ba/Zn stearate + melamine (4:1) phr |
40 |
Ca/Ba/Zn stearate + Ca/Zn phosphate (4:1) phr |
30 |
Ca/Ba/Zn stearate + Ca/Zn phosphate (3:2) phr |
48 |
Ca/Ba/Zn stearate + Mg(OH)2+ melamine (3:1:1) phr |
45 |
Ca/Ba/Zn stearate + ammonium poly phosphate+ melamine (3:1:1) phr |
48 |
Ca/Ba/Zn stearate + Mg(OH)2+ ammonium poly phosphate (3:1:1) phr |
43 |
Table 5 Stability time of prepared stabilizers against commercial one akropan 2611 px 5phr.
The degree of discoloration is related to the condition of exposure, such as length of period and temperature. When the conditions of exposure are fixed and controlled, then the relative resistance to discoloration due to heat of two or more compositions is able to be determined. The precision of heat stability testing is also dependent on the thickness of the specimens and the history of heat exposure prior to testing. This practice allows for control or the reporting of these variables. This practice is particularly applicable for determining gross differences in the heat stabilities of poly (vinyl chloride) compositions that are detectable as a color change. It is not intended to measure absolute thermal stability. Although the observed color changes may be evidence of degradation, molecular degradation phenomena such as chain-scission or cross-linking may not be identifiable. While discoloration caused by exposure to elevated temperature is commonly regarded as evidence of degradation in poly (vinyl chloride) compositions, this practice is able to predict the relative discoloration in processing, provided that the compositions in question are tested at the relative maximum temperatures developed in processing. This practice is not applicable to materials that will cross-contaminate during oven exposure.
From Static thermal stability results of the prepared PVC formulations we can conclude that:
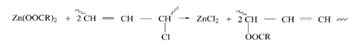
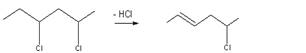
Evolved hydrochloric acid reacts with zinc stearate to produce zinc chloride according to the following equation
Also zinc stearate reacts with PVC defect sites to produce zinc chloride.
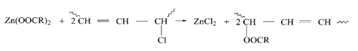
Zinc chloride is strong Lewis acid and catalyses dehydrochlorination process, but in the presence of calcium and barium stearates zinc chloride undergo ester exchange reaction, thus regenerating the zinc stearate which may react with another molecule from hydrochloric acid or PVC (this conclusion was reported by H. Ismet Gokcel et al).17
Calcium and barium chlorides are weak Lewis acids and would not catalyses' dehydrochlorination of PVC
Chemical structural, thermal and microstructure features have been supported by the central lab of the national research center, Dokki, Giza.
The author declares that there are no conflicts of interest.

©2019 El-Ghaffar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.