International Journal of
eISSN: 2475-5559


Research Article Volume 3 Issue 1
1Egyptian Petroleum Research Institute, AL-Ghad International College for Applied Medical Sciences, Kingdom of Saudi Arabia
1Egyptian Petroleum Research Institute, AL-Ghad International College for Applied Medical Sciences, Kingdom of Saudi Arabia
Correspondence: Sanaa M Solyman, Egyptian Petroleum Research Institute, AL-Ghad International College for Applied Medical Sciences, Kingdom of Saudi Arabia
Received: January 28, 2018 | Published: February 20, 2018
Citation: Solyman SM. Exposing kaolinite active sites and evaluating their activity in dimethyl ether preparation from methanol. Int J Petrochem Sci Eng.2018;3(1):33-38. DOI: 10.15406/ipcse.2018.03.00074
This study is an attempt to exposing kaolinite active sites and find out its catalytic efficiency in methanol dehydration to dimethyl ether (DME) using urea as a green chemical compound. The reaction was carried out at different reaction temperatures, different contact times and under atmospheric pressure. Natural kaolinite (KN) was treated with urea by two techniques which followed by boiling (U-K-B) or ultrasonication (U-K-US) in deionized water. These samples were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning and transmission electron microscopy (SEM and TEM), thermo-gravimetric analysis (TGA) and acid-base titration. XRD revealed that urea was intercalated in-between kaolinite layers and intercalation ratio is computed. FTIR and TGA confirmed that urea was eliminated from U-K-B but 3.3% still intercalated in U-K-US sample. TEM showed three steps for folding (nanoscrolling) of kaolinite monolayers. Although acid-base titration is a traditional method for evaluating Brønsted surface acidity but it is confirmed that U-K-B sample have higher acidity than U-K-US sample. So, urea-kaolinite complex which treated by boiling in water (U-K-B) have better performance in DME preparation with conversion of 87.5% and DME yield of 87.0% relative to sonicated sample (U-K-US).
Keywords: nanoscroll, kaolinite, characterization, dimethyl ether preparation
Dimethyl ether (DME) has commercial importance which is increased considerably in recent years. Diesel exhausts had been linked to cancer in a recent World Health Organization report,1 thus clean alternatives are desired.2 DME is extremely clean-burning and does not form peroxides as do higher ethers, and renewable and oxygenated alternative fuel for diesel engines.3 In addition DME is being considered as IGCC power generation,4 home fuel applications, and intermediate in petrochemical industry. Methanol dehydration to dimethyl ether over solid acid catalysts was first reported by Mobil in 1965. It is generally established that acid catalysts are the best materials for the dehydration of methanol to dimethyl ether and that either Brønsted or Lewis acids are capable for performing this reaction. However, most of these solid-acid catalysts produce undesirable side products, such as hydrocarbons and coke due to strong acid sites and higher dehydration temperature.5,6 Thus, extensive research has been focused on finding better catalysts which have higher selectivity for DME formation and fewer tendencies to generate hydrocarbons and coke.2 Several solid-acid catalysts such as HZSM-5, H-beta, alumina, and SAPOs were reported for methanol dehydration in a temperature rang of 250-400°C.7,8 Solyman et al.9 modified H-Beta and H-Mordenite using ultrasonication technique, which resulted in complete conversion of methanol with complete selectivity to DME at 125, 150, and 175°C under different contact times.
Kaolinite is a commercial solid acid catalyst which deactivates rapidly and has a tendency to generate hydrocarbon and coke, due to strong acid sites.10 If kaolinite is treated to be having benefit acidity for DME preparation, this will be a very important result. So, the performance of modified kaolinite in the present reaction had been previously studied via intercalation with alumina,11 chemical treatment by H2O2, and mechanochemical treatment by ball milling with and without CaSO4.12
Both kaolinite and urea are cheap, commercial, and save chemical compounds. Thus, the modification of kaolinite with urea to be used in the preparation of a clean fuel and fine chemical such as DME is a goal that has an economic benefit. So in the present study, the deep active sites of kaolinite were exposed through exfoliation and delamination to monolayers and nanoscroll, using urea via two methods. The effect of these modifications on kaolinite crystal structure was studied using XRD, FTIR, SEM, TEM, TGA, and acid-base titration techniques. Also, the catalytic activity of these modified kaolinite samples was evaluated in DME preparation through methanol dehydration reaction and correlated with their characterization results.
Kaolinite modification with urea
Exposing of kaolinite active sites was carried out through its exfoliation and delamination using urea by dynamic and static intercalation techniques as described in previous publication.13 The dynamic intercalation was accomplished via grinding of 10 g of natural kaolinite (KN) with 2.5 g of urea (20%) for 1h. The produced sample was treated via static intercalation technique by placing it in closed vessel and heating in a furnace at 95°C for 48 h, then was cooled and grinded. The resulted urea-kaolinite complex is denoted as (U-K). Urea–kaolinite intercalation was promoted by dividing this complex into two parts, the first part ultrasonicated in deionized water with heating to ~ 60°C (heat resulted from sonication) for 1 h, and the second part was boiled in deionized water for 1h. The two samples were washed and separated by centrifugation, then dried at 45°C for 48 h, and denoted as U-K-US and U-K-B, respectively. Ultrasonication was carried out with amplitude 60, cycle=1, and using a commercial sonicator VCX-750 (Sonics and materials, Inc) equipped with a titanium probe (diameter 13 mm), frequency of 20 KHz. These two catalyst samples were activated at 300°C in nitrogen atmosphere before the dehydration reaction.
Catalyst characterization
The two catalyst samples, U-K-B and U-K-US were characterized by XRD analysis using Bruker axs–D8 Advance CuKα target with secondary monochromator. Chemical structural features were revealed through FTIR measurements using Nicolet IS-10 FTIR over the wave number 4000-400cm-1. A JEOL JSM-5300 instrument working at 30 kV (SEM) was used for taking the scanning electron microscopy images. The instrument JEOL 2011 (Japan) electron microscope at 200 k V (TEM) was used for taking the transmission electron microscopy images. Thermo-gravimetric analysis (TGA) was carried out using the instrument SDT Q600 V20.5 Build15. Measurement of Brønsted surface acidity of the treated kaolinite samples was carried out by base exchange followed by acid titration using standardized 0.1M HCl and 0.1M KOH as follow:14 0.5g of the solid catalyst was dispersed in 120 ml KOH and agitated for 0.5 min, then settled for 1 min; 10 ml from this suspension was withdrawn and filtered. This step was repeated and 10ml was withdrawn after 1.5, 3.0, 4.5, 6.0, 7.5, 9.0, 10.5, 12.0, 15.5, 30.0, and 720 min; 1 min for sedimentation was allowed before each withdrawal which followed by filtration. 5 ml of the filtrate was titrated with 0.1M HCL using phenolphthalein indicator. Brønsted surface acidity was estimated as milliequivalent for 1g catalyst (meq/g).
Dimethyl ether preparation
The catalytic activity of U-K-B and U-K-US samples in vapor phase methanol dehydration to DME was evaluated. The reaction was carried out in a conventional flow type reactor12 at a reaction temperature ranged from 200 to 450°C; contact times of 15, 30 and 45 min, and a catalyst weight of 2.5g. The reaction products were analyzed using gas liquid chromatography (Hewlett Packard -5890) equipped with flame ionization detector and connected with Carbowax backed column.
XRD analysis of parent and treated kaolinite
Figure 1 show XRD patterns of U-K-B, U-K-US and natural kaolinite (KN) for comparison. The diffraction pattern of U-K-B sample shows a pronounced reduction in the intensity of all diffraction peaks which accompanied with broadening in 001 and 002 basal planes relative to its parent KN, which had been previously published.11 This indicates that urea is intercalated with kaolinite and leads to the breakdown of kaolinite crystals along the basal planes. Also, there are two broad peaks for 001 plane appear at 2θ o=12.34o and 10.55o corresponding to d-spacing of 7.17Å and 8.38Å. These results confirm urea intercalation in-between kaolinite layers. This intercalation may be accompanied with exfoliation/delamination which result in a new phase (H) that was confirmed by Valášková et al.15 The extent of urea-kaolinite intercalation was monitored using the intercalation ratio (IR) according to Wiewiora and Brindley equation.16 It is found that the IR ratio is 43% with d-spacing=8.38Å in sample U-K-B indicating incomplete exfoliation/delamination of kaolinite in U-K-B sample.17,18 On the other hand, the intensity of some diffraction peaks in U-K-US pattern is nearly similar to that of KN but more intense and sharp relative to U-K-B peaks. This may indicate that the sample U-K-US is more crystalline than U-K-B sample. Also, 001 plane have two peaks which appear at 2θo=12.32o (broad) and 8.27o (sharp), corresponding to d-spacing=7.18Å and 10.68Å, respectively. This is confirmed by computing the intercalation ratio (IR) which is increased to 71% due to ultrasonication in U-K-US sample relative to U-K-B (43%). The abovementioned results and the comparison between d-spacing of 001 plane for KN against low angle 001 planes for U-K-B and U-K-US (which are 7.13, 8.38 and 10.68Å respectively) mostly indicates the presence of nanoscroll in modified samples, more exfoliating and delaminating of kaolinite crystals in U-K-US relative to U-K-B and formation of a new phase H.12,17,18
FTIR- Spectroscopic analysis
FTIR spectroscopic analysis for both treated samples U-K-B and U-K-US are shown in Figure 2. This figure may indicate the presence of the inner-surface hydroxyl vibrations in which hydrogen is oriented towards the interlayer (at ~3697 and 3653 cm-1), and the hydrogen of the inner hydroxyls which oriented towards the vacant sites (at ~3620 and 912 cm-1).15 The intensity of these bands and the framework bands (from 400 to 1100 cm-1) is similar to those of parent kaolinite (inset in Figure 2) in U-K-B spectrum, but is increased in U-K-US spectrum. This finding may indicate more terminal AlOH and SiOH due to ultrasonication.12 Also, there are new bands appear in the spectrum of U-K-US sample as follow: strong band at 3503 cm-1 due to hydrogen bonding between NH2 and siloxane group (O-Si-O),15,18 two weak bands at 3417 and 3394 cm-1 due to symmetric and asymmetric stretching vibration of NH2 groups, which may interact with the oxygen of Si2O5 layer and the weaken hydrogen bonding between kaolinite layers,15 C=O stretching bands at 1675, 1622, and 1589 cm-1 and vibration band at 1477 cm-1 due to HNC deformation of urea molecules.19 These results mostly indicate the presence of urea which is intercalated with the tetrahedral sheet of kaolinite crystal. On the other hand, U-K-B have small and weak band at 3541 cm-1 and other weak and broad band at 1657 cm-1. This may be attributed to O-H stretching and binding vibrations of residual water which causes an increase of the interlayer space, even after drying for sample U-K-B.15 These results indicate that urea molecules may be not present in U-K-B sample.
Scanning Electron Microscopy (SEM) of treated samples
Figure 3 shows the morphology of treated urea-kaolinite complex samples by boiling (U-K-B) and ultrasonication in water (U-K-US). The figure shows the presence of very thin irregular kaolinite plates, scroll and curved plates in both samples but the ultrasonicated sample have more homogeneous morphology. The scrolling of kaolinite plates is not complete with all sheets mostly because of the small diameter of urea molecules and its low concentration in U-K complex (20% wt).20
Transmission Electron Microscopy (TEM) of treated samples
Figure 4 shows different micrographs for sonicated sample U-K-US. Figure 4A shows intercalated hexagonal kaolinite monolayers with different d-spacings, which have well-defined edges and corners.13 This indicates high degree of kaolinite crystallinity which is consistent with the XRD data. There are dark spots which may be urea or its trapped decomposition products such as NH3, HCN and CO2 as will be shown in the following discussion parts (Figure 4B). Also, nanoscrolls with different sizes and tubular form and monolayers folded surrounding each other are clear in Figure 4(C,D).21
Figure 5 shows TEM micrographs which indicate three steps of scrolling in U-K-B sample. The three micrographs show separated kaolinite monolayers due to their interaction with urea and then appear as a curve like tree leaf then scrolled as appeared in Figure 5A-5C.13 TEM images confirm partial exfoliation/delamination followed by scrolling and U-K-B sample is pure relative to U-K-US sample.
Thermo-gravimetric analysis (TGA) of treated samples
Figure 6 shows the Thermo-gravimetric analysis of U-K-B and U-K-US samples from 50 to 650°C. The total weight losing of U-K-B sample is 14.8% in two steps. The first step starts from 50°C to 418°C, due to the libration of physisorbed water and water of kaolinite crystallinity. The weight losing of this step is 3.5%. The second losing step starts from 418°C to 650°C due to the thermal dehydroxylation process.11 The weight loss of this step is 10.8%. On the other hand, the thermogram of second sample U-K-US shows three steps for weight losing, where the total mass losing value is 17.0%. The first mass losing step (5.1%) starts from 50°C to 221°C due to: 1) the libration of physisorbed water with weight loss of 0.9% from 50 to 133°C, 2) urea melting at 133°C up to 221°C and thus its decomposition in this range with weight loss of 3.3% and 3) libration of physisorbed water and water of crystallinity with weight losing of (~0.9%). The second mass losing 1.7% starts from 221°C to 418°C, which is the same value of the temperature range from the thermogram of U-K-B. These observations confirm that this weight losing may be attributed to the libration of physisorbed water and water of crystallinity, and that urea may react with the librated water and decomposes completely up to 221°C according to the following equation (eq. 1):
(NH2)2CO + H2O → 2 NH3 + CO2 ……………. (1)
The fourth mass losing step starts from 418°C to 650°C with weight loss of 10.2 % due to thermal dehydroxylation process such as in U-K-B thermograme. These results confirm the phenomenon that urea removed completely due to boiling in deionized water. On the other hand, urea weight% in U-K complex is 20%. So, it can be computed that 16.7% urea is removed during ultrasonication in deionized water at ~ 60°C and 3.3% still interacts with kaolinite layers in U-K-US sample. These results coincide with FTIR explanation and TEM images.
Brønsted surface acidity of the treated kaolinite samples
Figure 7 shows the estimated Brønsted surface acidity of treated kaolinite samples U-K-B and U-K-US by shaking with KOH solution followed by titration with HCl solution which results in three observations.
The first observation: the initial surface acidity values of U-K-B and U-K-US samples are 100 and 150 meq.g-1, respectively after 1.5 min. Brønsted surface acidity of kaolinite mostly promote the formation of hydrogen cyanide (HCN) which trapped on kaolinite surface in U-K-US sample. HCN is slightly acidic and may be responsible for the high initial acidity (150 meq./g) at 1.5 min.
The second observation: the surface acidity values change between decent and ascent after different time intervals of shaking and then are stabilized. This change may be due to interacted NH3 and CO2 molecules on acidic and basic sites, respectively of kaolinite surface. Literatures have been indicated that potassium hydroxide and other basic hydroxides can be used as adsorbents for CO2 in purification process for clay.22,23 So, a volume of KOH solution may be consumed by CO2 molecules according to the following equation and results in a change in the calculated acidity value:
2KOH + CO2 → K2 CO3 + H2O ……………… (2)
The third observation: the final surface acidity values of U-K-B and U-K-US samples are 200 and 110 meq.g-1 after 15.5 and 10.5 min, respectively (see Figure 7) and still stable up to 720 min. This result indicates that sonicated sample (U-K-US) mostly suffers dealumination which results in a decrease in its Brønsted surface acidity.14 It is concluded that the traditional acid –base titration technique give an important idea that U-K-B sample have higher Brønsted acidity relative to U-K-US sample.
The mechanism of kaolinite scrolling through urea-kaolinite intercalation
Figure 8 shows the exfoliation and delamination mechanism of kaolinite layers to separated and folded monolayer through its intercalation with urea as follow: urea molecules are diffused and intercalated in-between kaolinite layers by dynamic and static process due to grinding and heating in a furnace at 95°C, respectively producing urea-kaolinite complex. Part of this complex is treated with boiling water and the other part is treated by sonication in hot water.20 These treatments result in increasing the d-spacing values due to several factors:
The evaluation of modified kaolinite samples in dimethyl ether preparation
Exfoliation/Delamination of kaolinite particles exposes some active sites which mostly improve the catalytic activity of kaolinite. So, the performance of treated kaolinite samples has been evaluated by studying the conversion percent of methanol by its dehydration (conv.%) and the yield percent of dimethyl ether (Y%) using both treated samples, U-K-B in Figure 9 and U-K-US in Figure 10. The reaction temperature is ranged from 200 to 450°C at different contact times 15, 30, and 45 min.Generally, the two figures indicate that U-K-B is more active than U-K-US. This observation can be attributed to the final Brønsted surface acidity which is higher for U-K-B. The maximum conversion and yield % are 87.5 and 86.8 respectively at 400°C with contact time 15 min using U-K-B as shown in Figure 9, while by using U-K-US sample the maximum conv.% decreases to 45 and the maximum Y% decreases to 43.2 at 400oC and contact time of 30 min (Figure 10). By using U-K-B catalyst, the conv.% and the Y% at all reaction temperatures are increased relative to parent kaolinite (KN), as previously published.12 This means that the modification in sample U-K-B has been created or exposed moderate active sites (bridged silanol groups) which have better activity in methanol dehydration and selectivity to DME at low reaction temperatures.9,12 Also, more concentration of terminal silanol groups (strong active sites) has been created or exposed because the performance at high reaction temperatures i. e, the conv.% and the Y% are increased up to 400°C. Above 350 and 400°C with different contact times, the conv.% and the Y% are decreased mostly because of these strong active sites which promote olefin formation and coking (decomposition of methanol and olefins producing carbon) thus deactivate the catalyst at elevated temperatures and higher contact time (45 min) as shown in Figure 9.9,12
On the other hand, U-K-US sample has urea molecules which still present and intercalate with some kaolinite layers. Catalyst characterization documents that urea molecules are melted and mostly decomposed thermally to NH3 and CO2 in the reaction temperature range. These gases must be escaped at all reaction temperatures, so more active sites must be exposed which result higher activity relative to U-K-B sample. Figure 10 indicates the opposite, because the conv.% and the Y% are decreased with large extent relative to the results in Figure 9 using U-K-B catalyst. This means that the decline in the performance of U-K-US sample may be resulted due to some other reasons. Firstly; the presence of urea, ammonia, and CO2 mostly overcrowding the active sites. Secondly; thermal-catalytic oxidation of ammonia into nitrogen oxide and CO2 gases may be proceeds by kaolinite strong active sites during the reaction and also thermal-catalytic reduction of carbon dioxide into carbon. This carbon mostly poisoning the kaolinite active sites in U-K-US sample (IC=71%) with higher extent relative to U-K-B sample (IC=43%), and thus decreases its catalytic performance. These results are coincided with FTIR results which show a strong band at 912.8 cm-1 due to increasing the terminal silanol groups (strong acid sites).
It can be conclude that the present treatment of kaolinite by boiling U-K complex in water has been resulted in a better modification in kaolinite acidity in U-K-B sample with respect to sonicated sample U-K-US. Also, U-K-B catalyst give higher conversion% for methanol and better yield% of DME relative to other modification methods by H2O2, ball milling with and without CaSO4 which published previously.12
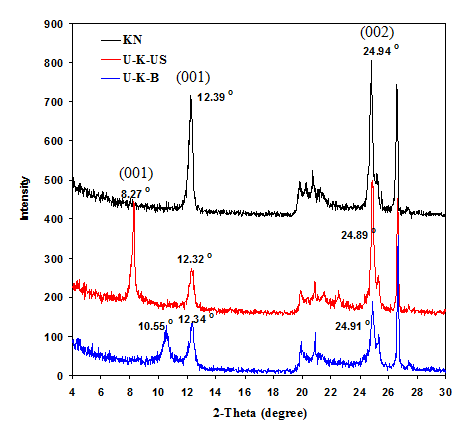
Figure 1 X-ray diffraction patterns of natural kaolinite (KN), sonicated (U-K-US) and boiled (U-K-B) samples.
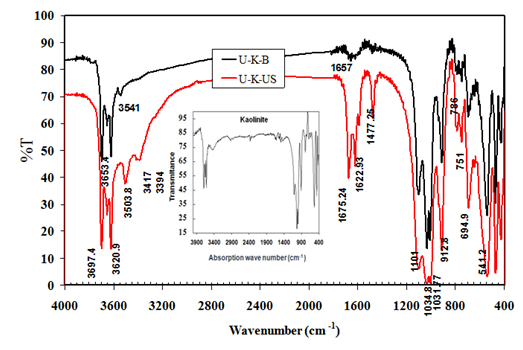
Figure 2 FTIR spectroscopic analysis of treated kaolinite samples (U-K-US) and (U-K-B). The inset spectrum is for natural kaolinite (KN).
The present work is a trial to study the performance of kaoliniteʼs exposed active sites in dimethyl ether preparation. This carried out by diffusion and intercalation of urea (U) in-between kaolinite layers (K) through two stages, and then promoted by boiling and by ultrasonication in deionized water to give samples (U-K-B) and (U-K-US). The produced samples are characterized by XRD, FTIR, SEM, TEM, and TGA techniques and acid-base titration. Each sample contains fine hexagonal (planner), curved and folded (nanoscroll) monolayers. Sample U-K-B has higher Brønsted surface acidity, mostly due to creation of moderate acidic sites. So, this sample has good performance and gives methanol conversion of 87.5% and DME yield of 87.0%. On the other hand, U-K-US sample have created terminal silanol groups which promote cocking. So, the performance of this sample is declined. It is concluded that kaolinite and urea are cheap and save chemical compounds, they can be used to prepare commercial, active, and selective catalyst (nanoscroll kaolinite), but higher concentration of urea can be used to give complete scrolling for kaolinite layers by boiling in water method.
The author declares no conflict of interest.

©2018 Solyman. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.