International Journal of
eISSN: 2475-5559


Research Article Volume 7 Issue 1
1Instituto Federal do Piauí, Brazil
2Instituto Federal de Sergipe, Brazil
Correspondence: Karine Oliveira Moura, Instituto Federal de Sergipe, Povoado Piabas, s/n, Zona Rural. Nossa Senhora da Glória, Sergipe, Brazil, Tel +55 79 999510796
Received: April 28, 2024 | Published: May 17, 2024
Citation: de Sousa JP, Silveira FF, Moura KO. Coconut fibers as a natural adsorbent to brilliant coomassie blue dye adsorption. Int J Petrochem Sci Eng. 2024;7(1):29-32. DOI: 10.15406/ipcse.2024.07.00140
The adsorption method is widely studied by the scientific community for the treatment of water containing pollutants, since water quality and its preservation is a current topic of great importance. Therefore, the present work analyzed the potential of coconut fibers as a natural adsorbent for the adsorption process of Brilliant Coomassie Blue dye solutions, using UV-vis spectrophotometry as a method to analyze the removal process. For this, the parameters of adsorbent dosage, initial concentration and contact time were evaluated. Furthermore, the kinetic study was carried out applying the pseudo-first order and pseudo-second order models. It was observed that coconut fiber is an easy-to-use material as an adsorbent, resulting in a maximum adsorbed amount of 3.04 mg g-1, with 83.5% being the highest percentage of removal. Adsorption reached equilibrium within 24 hours and the Pseudo-second order model fitted the process better, indicating chemical adsorption.
Keywords: adsorption, brilliant coomassie blue dye, coconut fibers, natural adsorbent, uv-vis spectrophotometry
The quality of water used for human consumption has deteriorated over the years. This is due to several factors, such as anthropogenic activities, population growth, unplanned urbanization, rapid industrialization, production of domestic wastewater and rapid geological, environmental and global change.1,2
Population growth and industrialization are mainly responsible for the increase in pollutants, such as heavy metals, pharmaceuticals, pesticides, among others, in the environment.3 There are several dyes used in industrial activities that, when released into the environment, cause an increase in pollution and pose a risk to human health, as is the case of Brilliant Coomassie Blue.4 Brilliant Coomassie Blue is a non-azo dye commonly used in the textile industries and gel electrophoresis, where its immense application is linked to its coloring and ease of use.5,6 Although it is a very useful material for the industrial sector, it is also an organic pollutant.
Pollutants can harm the aquatic ecosystem, since water contaminated by chemical compounds can travel long distances, causing risks to health, soil and aquatic plants.7 Water containing a dye such as Brilliant Coomassie Blue can cause damage to this ecosystem,8 since it is a carcinogenic, mutagenic, teratogenic and extremely toxic dye for respiratory treatment.6 Its presence can cause skin and eye irritation, impair photosynthesis in the aquatic environment by reducing the penetration of sunlight, in addition to leaving an unpleasant appearance in the environment.9 Due to water pollution, some methods of removing impurities are necessary to minimize contamination of these places.10
Currently, the adsorption method has been commonly used in water due to its efficiency, low cost, because it is a clean process and to remove pollutants in low concentrations, with low energy consumption.11 Various commercial adsorbents with high efficiency, such as activated carbon, carbon nanotubes and zeolites have been used. However, these materials have a high cost, making the search for low-cost and biodegradable adsorbents necessary.12 In this context, the use of agricultural waste as adsorbents has attracted the attention of the scientific community due to its abundance, economic principles and reuse.13,14 Therefore, the present work studied the use of coconut fibers as an adsorbent material to evaluate the process of removing Brilliant Coomassie Blue dye from astewater. For this, the effects of adsorbent dosage, initial adsorbate concentration and contact time, followed by the kinetic study, were studied.
Materials
The coconut fibers (Figure 1a) used in this work as adsorbent material were collected in the rural region of the city of Acauã, in the state of Piauí, Brazil. The fibers were manually separated, washed with tap water, dried naturally and stored in a dry environment for later use. The adsorbate used for this work was the Brilliant Coomassie Blue (C47H48N3NaO7S2) dye (Figure 1b) from Dinâmica Química LTDA.
Methods
The adsorption of Brilliant Coomassie Blue dye using coconut fibers as adsorbent material was studied by changing the factors of adsorbent dosage, initial dye concentration and contact time.
To evaluate the influence of the adsorbent mass on the dye adsorption process, a 15 mg L-1 solution was prepared. Then, 20 mL of this solution was added to Erlenmeyer flasks. In each of them, different amounts of adsorbents were added. The masses of the adsorbent used in the tests were 0.05, 0.15 and 0.2 g. To ensure the balance of the system, after 24 h aliquots of each of the solutions were collected for analysis. To analyze the effect of the initial dye concentration, dye solutions were prepared with concentrations of 2, 5, 10 and 15 mg L-1. Then, 20 mL of each solution was added to Erlenmeyer flasks to carry out the experiment. In each Erlenmeyer flask, 0.15 g of adsorbent was added. After 24 h, aliquots of the solutions were collected for analysis. To understand the effect of contact time, a 15 mg L-1 dye solution was prepared. Then, 20 mL of this solution was added to an Erlenmeyer flask. In each Erlenmeyer flask, 0.15 g of adsorbent was added. After predetermined times, aliquots of the solutions were removed to analysis. All processes were carried out in triplicate.
The aliquots collected were analyzed in the UV/VIS ESPEC-UV-5100, from Tecnal (Figure 2). Despite having low sensitivity, spectrophotometry in the UV-vis region has been widely used as a technique for determining the concentration of dyes in water,15–17 due to its low cost, ease of operating the instrument and quick determination of the measurement.18 Typically, a UV-vis spectrophotometer consists of a light source, which emits radiation for each of the UV and Visible regions, a monochromator, which scatters the emitted light for each region, and a detector.
The wavelength region for ultraviolet and visible spectrophotometry comprises the range from 190 to 800 nm.19 The wavelength used for the analyzes was 586 nm, close to that found in the literature.4
Figure 3 presents the image of the solutions before and after the Brilliant Coomassie Blue dye adsorption process when the dosage parameters (Figure 3a) and initial dye concentration (Figure 3b) were evaluated.

Figure 3 Brilliant coomassie blue solutions before and after the dye adsorption process using coconut fibers as adsorbent material, evaluating the parameters adsorbent dosage (a) and initial dye concentration (b).
(Figure 4a) and Table 1 presents the results relative to dosage of the adsorbent. It can be observed the relationship between the amount of dye adsorbed, when the mass of the adsorbent is modified, and the concentration of the solution is kept constant. To determine the adsorbed amount of dye at equilibrium (qe) and the removal percentage (%), Equations 1 and 2 were used.
(1)
(2)
|
Adsorbent mass (g) |
Adsorbed amount (mg g-1) |
Removal percentage (%) |
|
0.05 |
3.04 |
52.94 |
|
0.15 |
1.5 |
78.52 |
|
0.2 |
1.2 |
83.5 |
Table 1 Effect of the adsorbent dosage on the adsorption of the brilliant coomassie blue dye using coconut fibers as adsorbent material
Where qe is the amount adsorbed at equilibrium (mg g-1), Co and Ce are the initial and equilibrium concentrations of the Brilliant Coomassie Blue dye solution (mg L-1), respectively, V represents the volume of the solution (L) and M the mass of the adsorbent (g).20
The results relative to the variation in the initial concentration of the solution are represented in (Figure 4b) and Table 2. The data obtained were calculated using Equations 1 and 2.
|
Initial concentration (mg L-1) |
Adsorbed amount (mg g-1) |
Removal percentage (%) |
|
2 |
0.15 |
67.65 |
|
5 |
0.46 |
76.43 |
|
10 |
1.06 |
82.81 |
|
15 |
1.6 |
81.5 |
Table 2 Effect of initial concentration on the adsorption of the brilliant coomassie blue dye using coconut fibers as adsorbent material
The results presented in (Figure 4c) correspond to the study of dye adsorption in a determined time interval. The study was carried out with solutions at constant concentration (15 mg L-1), using 0.15 g of adsorbent. Data were analyzed using Equation 2 and 3.
(3)
Where Co and Ct are the initial concentrations and at time t (mg L-1), V is the volume of the solution (L), and M is the mass of the adsorbent (g).20
The kinetic study was carried out with data obtained from the study of contact time, with the application of the theoretical models of Pseudo-first order (Equation 4) and Pseudo-second order (Equation 5).
(4)
(5)
Where qe and qt represent the amounts adsorbed at equilibrium (mg/g) and at time t, k1 (h-1) and k2 (g/mg.h) are the first and second order rate constants, respectively, and t represents time (h).21
From the application of the Pseudo-first order and Pseudo-second order equations to the contact time data (Figure 4c), the results in (Figure 4d) and Table 3 were obtained.
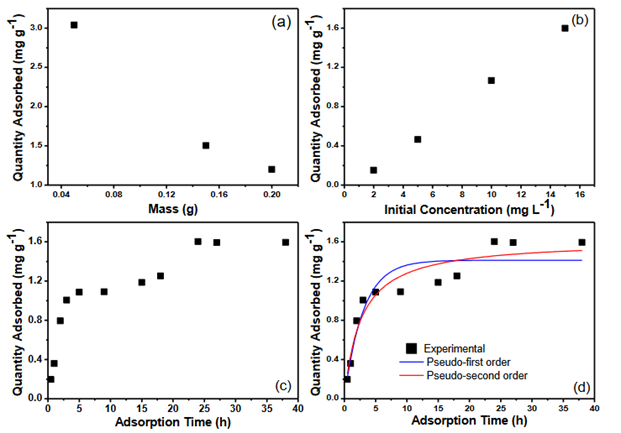
Figure 4 Influence of adsorbent dosage (a), initial dye concentration (b), variation in contact time (c) and kinetic study (d) on the adsorption process of the Brilliant Coomassie Blue dye using coconut fibers as adsorbent material.
|
Model |
R2 |
k |
qe |
|
Pseudo-first order |
0.86695 |
0.33361 |
1.41 |
|
Pseudo-second order |
0.91459 |
0.23349 |
1.62 |
Table 3 Parameters of the kinetic models for the adsorption process of the brilliant coomassie blue dye using coconut fibers as adsorbent material
It is possible to observe in Figure 3 that the adsorption process occurs significantly, since there is a difference in the color of the solutions for both parameters analyzed, observing translucency in the solutions of some Erlenmeyer flasks, that indicating almost complete adsorption of the Brilliant Coomassie Blue dye.
Considering the effect of adsorbent dosage, (Figure 4a) and Table 1, it is possible to observe that when increasing the mass of the adsorbent, the value of the adsorbed amount (qe) decreased. This phenomenon suggests that, as the mass of the adsorbent increases, the number of active adsorption sites also increases, thus causing a competition between the active sites to remove the dye, which results in a decrease in the qe.22 Table 2 also shows the percentage of removal of the Brilliant Coomassie Blue dye using coconut fibers as adsorbent. It can be observed an elevated with the increase in adsorbent mass, which can be explained due to the increase in the adsorbent surface and the greater accessibility of active adsorption sites.23
Regarding the effect of the initial concentration of the adsorbate, it is possible to observe in (Figure 4b) and Table 2 an increase in the adsorbed amount of the dye with the increase in the concentration of the solution. This happens due to the fact that, the higher the dye concentration of the solution, the greater the amount of adsorbate in the medium, that will eventually be adsorbed.24 It is also possible to note that the removal percentage, Table 2, becomes greater with increasing dye concentration, except when the concentration goes from 10 to 15 mg L-1, reaching removal percentages of 82.81 to 81. 50%, respectively, suggesting stability. This may have occurred because at lower concentrations of adsorbate, there is a greater availability of adsorption sites for these molecules. However, when increasing the amount of adsorbate in the solution, the active sites on the surface of the adsorbent tend to saturate, resulting in a reduction in the removal percentage.25
When evaluating the effect of contact time, (Figure 4c), it was possible to observe that the amount adsorbed increased rapidly in the initial times, until the process began to slow down. This can be explained due to the large number of active sites present on the surface of coconut fibers, which is reduced over time. Furthermore, the data demonstrate that adsorption reached equilibrium within approximately 24 hours. This long equilibrium time can be explained by the repulsion between the bulk adsorbate molecules and the solid phase.23 The maximum adsorbed amount of Brilliant Coomassie Blue dye on coconut fibers was 1.60 mg g-1.
To understand the adsorption mechanism, Pseudo-first order and Pseudo-second order models were applied to the adsorption data of Brilliant Coomassie Blue dye on coconut fibers. Observing the data obtained in (Figure 4d) and Table 3, it is clear that the Pseudo-second order model is the most appropriate to describe this adsorption process, since the correlation coefficient, R2, of the Pseudo-second order model presents a higher value when compared to the Pseudo-first order model. This result is similar to some data found in the literature.4,8 Since chemisorption is the limiting step of the Pseudo-second order model,26 it is suggested that the adsorption process for this work is characterized by chemical adsorption, involving chemical reactions between the dye particles and the functional groups present on the surface of the adsorbent.4
In this work, the adsorption process of the Brilliant Coomassie Blue dye was studied using coconut fibers as adsorbent material and Uv-Vis spectrophotometry as a data analysis technique. For adsorbent dosage, a higher percentage of dye removal (83.5%) was observed when 0.2 g of adsorbent mass was used, while a greater adsorbed quantity, 3.04 mg g-1, was found when the mass of 0.05 g was used. Furthermore, it was observed that, when increasing the dye concentration from 2 to 15 mg L-1, the amount adsorbed also increased from 0.15 to 1.59 mg g-1, reaching removal percentages of 82.81 and 81.50% for concentrations 10 and 15 mg L-1, suggesting stability. The dye adsorption process reached equilibrium in approximately 24 hours, with the Pseudo-second order model best suited to this process, characterizing the process of removing the Brilliant Coomassie Blue dye from coconut fibers as chemical adsorption. Coconut fiber is an adsorbent that is easily accessible and applied, providing good results in the adsorption process. The data obtained shows that coconut fiber has a high adsorptive capacity. In this way, the effluent can be treated with this agricultural residue, adding value to a byproduct that is generally discarded and not reused, contributing to recycling and environmental preservation through sustainable practices.
The authors would like to thank the Instituto Federal do Piauí, Campus Paulistana, for the physical and material support.
The authors declare that there is no conflicts of interest.
None.

©2024 de, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.