International Journal of
eISSN: 2475-5559


Short Communication Volume 2 Issue 2
1Chongqing Key Laboratory for Clean Energy and Resource Utilization, China
1Chongqing Key Laboratory for Clean Energy and Resource Utilization, China
2College of Chemistry and Chemical Engineering, Chongqing University, China
2College of Chemistry and Chemical Engineering, Chongqing University, China
3College of Chemistry and Chemical Engineering, Yangtze Normal University, China
3College of Chemistry and Chemical Engineering, Yangtze Normal University, China
Correspondence: Changyuan Tao, Chongqing Key Laboratory for Clean Energy and Resource Utilization, College of Chemistry and Chemical Engineering, Chongqing University, China
Received: February 13, 2017 | Published: March 3, 2017
Citation: Peng H, Liu Z, Tao C. Chaotic phenomenon in vanadium redox flow battery. Int J Petrochem Sci Eng. 2017;2(2):48-50. DOI: 10.15406/ipcse.2017.02.00031
chaotic phenomenon, Energy, ion-exchange, electrochemical, water, chemistry
Vanadium redox flow battery is an efficient energy-storage system because it’s long cycle life, flexible design, high efficiency and fast response time. Chaotic phenomenon (electrochemical oscillation) was first observed during the charging process of the anolyte, and the mechanism was investigated. The largest Lyapunov exponent was applied to characterize the chaotic phenomenon.
Vanadium and its compounds are used for manufacturing iron1-3 steel non-ferrous metals4 catalyst,5,6 and petrochemicals because its excellent properties.7,8 The vanadium sulfates were applied in vanadium redox flow batteries which proposed by Skyllas-Kazacos et al.9,10 in 1985. It was an efficient energy-storage system and had attracted much attention for its long cycle life, flexible design, deep-discharge capability, low pollution emitting and fast response time.11,12 The vanadium redox flow batteries were divided into two compartments by an ion-exchange membrane. The positive half cells was V(IV)/V(V) redox couples and V(II)/V(III) as the negative half cells. The electricity energy was transferred to chemical energy and stored in the vanadium compounds during the charging process. Studies were most focused on the materials of electrodes,13-15 and modification of membrane .16-19 Few studies had reported the electrochemical behavior of anolyte during the charging process.
All chemicals were analytical grade. Water was purified with a water purification system (HMC-WS 10, Korea). The anolyte was composed of VOSO4 (1 M) and H2SO4 (2 M), and the catholyte was H2SO4 (2 M) with the same volume. The anolyte and catholyte was separated with a Nafion membrane. The anode and cathode used in the experiments was platinum slice (1.5x2 cm) in which no other unexpected side reaction occurred. They were subjected to ultrasonic cleaning in de-ionized water for 30 min to remove residues before testing. Electrochemical tests were carried out on CHI660D electrochemical workstation. The signal of the potential was amplified, and the times series were recorded and transferred to a personal computer. The macro-instability frequency, oscillation period, of the potential-time series were obtained using Matlab.
Electrochemical oscillation was observed during the charging process, while the electrochemical tests were carried out on CHI660D electrochemical workstation with the anolyte of 1 M VOSO4 + 2 M H2SO4, and the catholyte of 2 M H2SO4 at room temperature, the potential-time series was showed in Figure 1. As indicated in Figure 1 the potential was increased quickly and then electrochemical oscillation emerged. After then, the period of the electrochemical oscillation slowly decreased and then stabilized as showed in Figure 2.
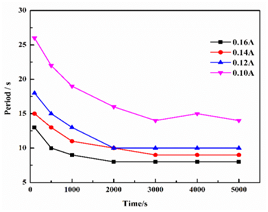
Figure 2 The dependence of oscillation period on the time in different charging current (anolyte: 1 M VOSO4 + 2 M H2SO4; catholyte: 2 M H2SO4).
The power consumption was investigated to reveal the influence of electrochemical oscillation on the charging efficiency. An extra power consume was a result of the electrochemical oscillation. The relative power-consume (PRC) was calculated as PRC= the average power consumption on anode / (Va·Ia), where Va was the potential, Ia was the charging potential. The results were shown in Figure 3. The influence of electrochemical oscillation on the charging efficiency will be enlarge in long-term charging, and much extra power will be consumed. This part of power consumption should exist in practical charging process, even though it is often ignored. It is beneficial for improving the charge efficiency and energy-saving if the electrochemical oscillation could be regulated.
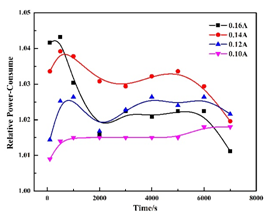
Figure 3 The dependence of relative power-consume on the time in different charging current (anolyte: 1 M VOSO4 + 2 M H2SO4; catholyte: 2 M H2SO4).
The mechanism of the electrochemical oscillation was investigated. During the charging process, the main chemical reactions in the system were described in Equation (1) and (2)
Anode: VO2+ + H2O - e → VO2+ + 2 H+……….. (1)
Cathode: 2H+ + 2e → H2……… (2)
The VO2+ in the anolyte was oxidized to VO2+ on the surface of the anode. As V(V) had low solubility in high acidic solution20-22 VO2+ would hydrolysis and formed V2O5 according to Equation (3).22 And also as the high acidic, v2O5 then dissolved according to Equation (4). The reaction showed in Equation (3) and (4) occurred alternately in the anolyte. The competition between the generation and dissolution of v2O5 resulting the electrochemical oscillation.23 The H+ in the anolyte would migrate to the cathode through the Nafion membrane. As the reaction happened like Equation (2), the H+ in the system was decreased, the solubility of V(V) increased21 and reactions showed in Equations (3) and (4) was interrupted, and then the electrochemical oscillation was disappeared. The reaction could be expressed like in Figure 4.
2 VO2+ + H2O → v2O5 + 2H+…….. (3)
v2O5 + H+ →2 VO2+ + H2O………. (4)
The electrochemical oscillation was an interesting self-organizing phenomenon in which the current or potential periodically fluctuates at a constant external electric field.24,25 It was also a typical chaotic phenomenon with non-linear and non-equilibrium. The main parameters to characterize chaotic phenomenon include Lyapunov exponent, correlation dimension and Kolmogorov entropy etc.26 Lyaponov exponent denotes the convergent or divergent average exponential rate of the adjacent orbits in the phase space of the system, and the value of the largest Lyapunov exponent (LLE) also apply to judge whether a nonlinear time’s series is in chaos or not.26-28 The LLE of the potential-time series was calculated using Wolf algorithm and the results were shown in Figure 5.
Figure 5 indicated that the LLE was positive for all experiments, which was consistent with the results that the electrochemical oscillation was a typical chaotic phenomenon. The LLE initially increased and then decreased with the electrochemical oscillation emitted. And it could be inferred that LLE would be decreased to below zero when the electrochemical oscillation disappeared from Figure 5.
In summary, the electrochemical oscillation was first observed during the charging process of anolyte in vanadium redox flow battery. It could be explained in term of the competition between the growth and the chemical dissolution of v2O5 film around the anode in the H2SO4 solution. The controlling and usage of the chaotic phenomenon was beneficial for improving the charge efficiency and energy-saving as the oscillation regular extra power consumption.
This work was supported by the Nation 973 projects of China (No. MOST2011CB933300) the Natural Science Foundation of China (No. 51274261) and the program for Chongqing University Postgraduates’ Innovation Project (CYB15045).
The author declares no conflict of interest.

©2017 Peng, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.