International Journal of
eISSN: 2475-5559


Research Article Volume 1 Issue 3
1Department of Chemical and Petroleum Engineering, UAE University, UAE
1Department of Chemical and Petroleum Engineering, UAE University, UAE
Correspondence: Nayef Ghasem, Department of Chemical and Petroleum Engineering, UAE University, UAE
Received: August 16, 2016 | Published: November 29, 2016
Citation: Ghasem N, Marzouqi MA, Rahim NA. Removal of cadmium from industrial wastewater using water-soluble polymer via hollow fiber membranes. Int J Petrochem Sci Eng. 2016;1(4):88-90. DOI: 10.15406/ipcse.2016.01.00016
The removal of toxic and polluting metal ions from industrial effluents, water supplies, as well as mine waters is an important challenge to avoid one of the major causes of water and soil pollution. To enable further processing and achieve recovery of metals, separation should be selective. One separation technique, which can meet this requirement, is polymer-enhanced ultra filtration (PEUF). Ultra filtration membranes would only retain macromolecular solutes. PEUF uses water-soluble polymeric agents to complex easily interesting metallic ions and give them a macromolecular size. Binding of metal ions to water soluble polymers enables us to separate them from solution and retain them when they are pumped through an ultra filtration membrane. In the present work Polyetherimide (PEI) is used as water soluble polymer for removal of cadmium ions from waste water via hollow fiber membrane contactor module. Polyvinylidene fluoride (PVDF) hollow fiber membrane contactor is used for this purpose. The PVDF fiber was custom made using thermal induced phase separation techniques. Experimental observations reveal that when PEI concentration increases the percentage removal of Cd (II) also increases. The molecular weight of PEI has positive impact on Cd (II) retention. The pH value of feed has effect on Cd (II) withholding. The percentage of removal increases with solution pH value. Recycling either retentate; the part of a solution that does not cross the membrane; or permeate have no effect on removal. Increase of mixing time before operation increases metal ions removal rate.
Keywords: Cadmium ions; Waste water; Membrane Contactor; Ultra filtration; PEI
The removal of toxic and polluting metal ions from industrial effluents, water supplies, as well as mine waters is an important challenge to avoid one of the major causes of water and soil pollution. To enable further processing and achieve recovery of metals, separation should be selective. One separation technique, which can meet this requirement, is polymer-enhanced ultra filtration (PEUF). Ultra filtration is a membrane technique working at low trans membrane pressures, which doubtless reduces operation costs. Nevertheless, ultra filtration membranes would only retain macromolecular solutes. PEUF uses water-soluble polymeric agents to complex easily interesting metallic ions and give them a macromolecular size. Binding of metal ions to water soluble polymers enables us to separate them from solution and retain them when they are pumped through an ultra filtration membrane.1
Water treatment plants use several different purification methods in order to thoroughly clean their water. The most common of these methods are: oxidation, reduction, precipitation, filtration and the use of bacteria. Unfortunately, these methods are not always effective for the sufficient removal of heavy metal ions. Membrane separations are efficient and widely applied separation processes that are comparable to other separation techniques in terms of technical and economic feasibility.2 The main disadvantage in using membrane processes for treatment of effluents with heavy metals is the size of the dissolved metallic salts. These hydrated ions or low molecular weight complexes, pass easily through most membranes with the exception of reverse osmosis and Nano filtration membranes. However, as these membranes are relatively non selective, all the metallic ions are retained together with alkaline and alkaline-earth ions.3
Selective separation can be achieved by a number of methods that selective bind metals to large polymeric or aggregated materials such as liquid micelles that contain selective ligand groups that can bind the metals and can be used in combination with membrane filtration.3 A number of interactions are possible but one of the most useful is that of chelation. A soluble polymer reagent with chelating groups is characterized by two main components: the polymer backbone, which provides the solubility and stability of the reagent, and the functional groups, which are necessary for the selective reactivity of the polymer.4
Polyetherimide (PEI) is very interesting compound to study. Commercial branched chained PEI has primary secondary and tertiary amino groups in a 1:2:1 ratio. The branched forms are able to form stable complexes with PEI and these properties have been described.3 Thus the combination of the two phenomena; binding of metal ions to a water soluble polymer, and ultra filtration is commonly termed Polymer Enhanced Ultra filtration (PEUF). This enables the separation of metal ions bound to soluble polymers from non-bound metals by use of an ultra filtration membrane. This forms a permeate solution almost free of metals and a retentate with a high metal-complex content.1
There are many potential advantages of using soluble polymers for metal removal in that, unlike ion exchange resins, they have very good kinetics of adsorption as there are little or no mass transfer limitations associated with the polymer,5 so allowing rapid absorption and maximal utilization of the absorbent capacity of the polymer. The drawback of using the PEUF is that the polymer normally interacts with the membranes. The nature of this interaction is dependent on the solution conditions, particularly ionic strength and most critically the pH, which can alter both the membrane charge and the net charge on the polymer. The overall result of the interaction of the polymer with the membrane is to cause fouling and increased membrane resistance, which results in a reduced flux.3 The main objectives of this research are to study the performance of PEI as binding polymer for removal of Cd (II) and investigation of the effects of pH, equilibrium time, and other parameters on the removal efficiency.
Materials
The chemical reagents used in the experiments were Polyetherimide (PEI) with a MW of 20,000 and 70,000 as a polymer binding solutions, cadmium nitrate tetra hydrate for preparation of Cd (II) solution, hydrochloric acid, and sodium hydroxide for pH adjustment. All the chemicals were used without treatment. Deionized water obtained from deionization system was used for dilution and preparation of feed solution. The membrane used was Minntech FiberFlo polyphen polysulfone membrane cartridge (M-I-050-A) with membrane surface area of 2 ft2 and pore size 0.05 μm.
Apparatus
The system employed is shown in Figure 1. The process contains the following items: feed tank, retentate, and permeate reservoirs with tubing and backpressure valve; a peristaltic pump, stirrer for mixing. A HANNA pH meter was used for pH measurements. The concentration of Cd (II) was determined using a equipment.
Experiment
The feed consists of a 10 ppm Cd(II) solution containing the desired amount of water soluble polymer at various pH valued adjusted to the desired value using either HCl or NaOH. In order to study the mass effect, the amount of the polymer varied from 5g/l to 20g/l keeping the total Cadmium concentration equal to 10 ppm and solution pH at desired optimum value. All experiments were conducted at room temperature. In all experiments, the feed volume was 500 cm3 and the first 10 cm3 of permeate was discarded. The feed solution is kept stirred at 200 rpm and circulated by means of the pump, then passed through the membrane cartridge. The retentate and permeate streams, and the feed were then analyzed for their Cd (II) content. The retention values were calculated from the formula:
Where Cf is the concentration of metal ion in permeate, and Cf is the concentration of metal ion in the solution of the feed stream. The used membrane was immediately flushed with de-ionized water after ultrafiltration (UF). Samples were analyzed using a Varian ICP-AES equipment.
The effect of PEI concentration on Cd(II) retention
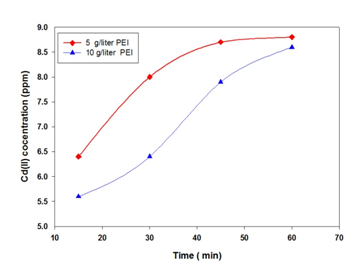
With initial feed concentration of 10 ppm Cd(II) in water. A polymer concentration of 5 g/liter and 10 g/liter PEI is used for the removal of Cd ions from water. The PEI polymer molecular weight is 20,000. The Cadmium concentration at two different PEI concentrations is shown in Figure 2. The results revealed that increase in PEI concentration results in a decreased in Cd concentration of the permeate stream. This is attributed to more probability for Cd to be attached with the concentrated PEI. With time the concentration of Cd ions in the permeate increased due to increased membrane resistance due to polymer accumulated on membrane pores. Figure 3 shows the percent removal rate of Cd from water using PEI polymer. The results revealed the percent removal decreased with increased time. This is attributed to increase in membrane resistance and the less chance for metal ions to be hocked with polymer functional group.
Effect of PEI molecular weight on Cd(II) retention
The effect of PEI molecular weight on Cd concentration in the permeate stream is investigated at two polymers molecular weight. The resulted shows that the higher the PEI molecular weight results in improved Cd removal rate Figure 4. The larger the molecular weight the larger the polymer size and hence the increase in the chance of metal ions to be attached with the PEI amine functional group.
The effect of pH on Cd(II) retention
Solution pH values have strong impact on Cd removal rate Figure 5. As the pH value increased, where the solution becomes more basic the percentage removal of Cd increased. The PEI affinity to capture Cd improved with increased pH value of the solution. A monotonic increase in Cd percent removal was observed after pH=7. The more basic the solution the better the percent removal and the lower the Cd concentration in the permeate stream.
Effect of mixing time
The effect of mixing time of prepared feed is shown in Figure 6. The increased in mixing time gives more time to PEI polymer and Cd(II) to make more bonds and hence when separated better removal rate is achieved with feed with high mixing time.
Polyetherimide (PEI) was used as water soluble polymer to enhance cadmium ions removal through ultra filtration process via hollow fiber membrane contactor made of PVDF hollow fibers. The effect of PEI concentration, PEI molecular weight, and solution pH and solution preparation mixing time were investigated. It was observed that when PEI concentration increases the percentage removal of Cd (II) also increases. The molecular weight of PEI has positive effect on Cd (II) retention. The pH value of feed solution has effect on Cd (II) retention. The higher the pH values the increased in the Cd removal rate. The percentage of removal of Cd(II) increases with solution pH value. The effect of solution mixing time before the start of the operation was investigated and was found to have strong impact on separation performance. The percent removal of cadmium ion increased monotonically with mixing time.
None.
The author declares no conflict of interest.

©2016 Ghasem, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.