International Journal of
eISSN: 2574-9889


Review Article Volume 5 Issue 6
West School of Medicine, University of Chile, Chile
Correspondence: Elba Wu Hupat, Professor West School of Medicine, University of Chile, MD, Miraflores 686, Appt. 501, Santiago, Chile
Received: November 26, 2019 | Published: December 24, 2019
Citation: Wu E. HIV/AIDS in children, Chilean experience. Int J Pregn and Chi Birth 2019;5(6):232-239. DOI: 10.15406/ipcb.2019.05.00183
The magnitude of the problem is described worldwide and in Chile. It is highlighted that globally, measures to prevent HIV transmission in both adults and pregnant women and the growing increase in people accessing ART, HIV infection in adults, but especially in children, has been halted and even reduced. However, in Chile, in the last 10 years there has been an increase in total cases, especially in the age of procreation. Exception is infection in VT infected children that has decreased to <2%. It describes, (a) how HIV exposed/infected newborns and children already infected with HIV can be detected and which flow-chart to follow; b) HIV VTPPs components; c) how to certify HIV infection in newborns and children under 18 months of age, due to the presence of maternal passive antibodies, which may last up to 18 months, the HIV/DNA/PCR technique should be used; (d) the study/evaluation and preventive and therapeutic management of HIV-exposed newborns and already infected children.
With regard to HIV infection in children and adolescents in Chile, the creation in 1990 of the Committee on Pediatric HIV/AIDS, which works together with MINSAL in the Pediatric HIV/AIDS Care Programme, stands out. The Committee consists of representatives from all regions of the country and its main objectives have been to detect for newborns/children exposed to HIV (children of HIV+ mothers) and children already infected with HIV, and to follow up (assessment/study, preventive and therapeutic management) of them up to the age of 18 years, the age at which they are transferred to the Adult HIV Programme. In Chile, the identification of HIV-infected children has been increasingly earlier in life, so has their access to ART. In the exposed newborn, the HIV/DNA/PCR technique began to be applied in 1992, lowering the HIV certification age from more than 20 months to 3 to 4 months. HIV VTPPs began to apply in 1995, down VIH VT from >35% before that year to 2% in 2005. With the promulgation of the HIV Prevention Norm by MINSAL in 2005, HIV VT has declined to <2% today. With the identification of infected children increasingly earlier in life, in less severe clinical and immunological stages, improvement in preventive and therapeutic measures, with greater access to ART, has been achieved to prolong survival and decrease lethality; 70% of children infected with HIV VT are in adolescence or adulthood, and some women have had uninfected children thanks to HIV VTPPs use.
Keywords: immunodeficiency, antiretroviral therapy, cytomegalovirus
AIDS, acquired immunodeficiency disease syndrome; ART, antiretroviral therapy; ARV, antiretroviral; HIV, human immunodeficiency virus; VL, viral load; VT, vertical transmission; VTPP, vertical transmission protocol; MINSAL, ministry of health; CMV, cytomegalovirus; STD, sexual transmitted diseases; TORCH, toxoplasmosis-other-rubella-cytomegalovirus-herpes.
Magnitude of the problem and transmission mechanisms
The first cases of HIV/AIDS in adults were described in the world in 1981 and the first cases in children in 1982, acquired by transfusion, and in 1983, by vertical transmission (VT), mother to child. Since then and until the end of 2018, worldwide, 74.9 million people had acquired the infection and 32 million had died from AIDS-related illnesses. By the end of 2018, of the 37.9 million people worldwide living with HIV, 1.7 million were <15 years, a figure that was previously > 2 million.1
In Chile, the first cases of HIV/AIDS in adults were described in 1994 in men and in 1995 in women. The first cases of HIV/AIDS in children were described in 1987 (by transfusion) and in 1989 (vertical). A total of 47567 cases (39946 men and 7621 women), of them 442 children (408 per VT, 34 from other causes), have been reported from the earliest cases described in adults and from the first cases described in children, through the end of 2018.2–4 In adults the main route of HIV transmission is sexual, with a progressive increase in heterosexual pathway. In children, the main route of HIV acquisition is by VT (95%) and less frequently, by horizontal transmission [contaminated blood transfusions or blood products, transplant of infected organs, intravenous drug, piercings, sexual abuse, etc.].5
As a result of the increase in heterosexual transmission in adults and, to a lesser extent, of the rise in drug-addicted women, then occurred globally, including Chile, a rapid and sustained increase in cases of HIV / AIDS in women (feminization tendency). Currently most infected adults are of childbearing age and nearly half of adults-of-childbearing age are women. This has resulted in an increase in children exposed to HIV, which initially meant a rise in HIV-infected children.1,6–12
However, thanks to the implementation since 1994 of prevention measures of VT of HIV, this increase in HIV in children, worldwide, has been able to stop and even reversed as more sites adopt prevention measures.13–18 From the moment that worldwide have been implementing measures to prevent the transmission of HIV in adults and in pregnant, and has increased the number of people with access to antiretroviral therapy (ART) (about 54% % of adults and 43% of children under 15 years had access to ART by 2016), the number of new people infected has been declining and the number of people living with HIV/AIDS has increased thanks to the longer survival of people accessing to ART. According to UNAIDS, by the end of 2010, 48% of pregnant women living with HIV had access to antiretroviral drugs (ARVs) which rised to 62% in 2012 and in many countries the coverage rate was above 80%. Since 2001 there has been a worldwide reduction in new HIV infections by nearly 40% in adults and 58% in children. From 2010 to 2018, new HIV infections decreased 16% in adults and 41% in children. Since 2004, the year with the peak of deaths, HIV-related death has been reduced by >55%. AIDS mortality has dropped 33% since 2010.1,6–12,16– 18
In 2018, of all people living with HIV, 79% were aware of their HIV status, of these, 78% had access to HIV treatment, and of these 86% had achieved an undetectable viral load (VL).1 In children it is where we have seen the greatest reduction of infected cases worldwide. Thanks to the progressive implementation of HIV Vertical Transmission Prevention Protocols (VTPPs), there has been a significant reduction (58%) of new infected children by VT between 2000 and 2014, with 47% since 2010 to the 2016. To this decrease in new HIV infections in children has contributed the increased access to treatment of the pregnant women.1,6–13,16–18
HIV VT can occur at any period of pregnancy (through the placenta), during labor (maternal-fetal blood transfusion), during childbirth (by HIV infected blood, secretions or fluids present in the delivery channel) or postnatally (breast milk intake), being the most common late transplacental and intrapartum transmission (50-70%); transmission during pregnancy is less frequent (30-50%) and postnatal rare (19). The risk that a woman infected with HIV transmit the virus to the child in pregnancy, if no preventive measures are taken, varies between 15 and 45%, and most often about 25 to 35%. Figures in these ranges were observed in Chile before incorporation of HIV VTPPs with ARVs.1–4 In the VT the virus present in the mother infected blood, amniotic fluid, secretions or breast milk, would penetrate to the gestation product through skin breaks or through the digestive tract.
The VTPP of HIV include, in the pregnant woman detect if she is infected with HIV (offer test), if infected, offer HIV VT preventive ART and / or therapy of their HIV infection, plus other preventive measures such as elective caesarean (childbirth could be vaginal only if the VL is undetectable or <1000 copies / mL at week 34, ART was initiated at week 20 or before, gestational age is 37 weeks, the fetus is unique, in cephalic presentation, and with favorable obstetric conditions) and membrane rupture is not more than 4 hours; in the newborn, replace breastfeeding for infant formula, and follow with the protocol of preventive ARVs for 4-6 weeks.16,20,21 The use of ARVs in pregnant has gone of monotherapy (zidovudine, Protocol PACTG-Pediatric AIDS Clinical Trials Group- 076 original of 1994, with use of oral zidovudine during pregnancy, injectable during delivery and oral in the newborn), to use in the woman of bi-therapy (zidovudine+lamivudine) during gestation and subsequently of triple therapy (backbone of two nucleoside reverse transcriptase inhibitor, zidovudine or abacavir, plus a third ARV that may be a protease inhibitor, usually lopinavir / ritonavir, or a non-nucleoside reverse transcriptase inhibitor such as nevirapine or an integrase strand transfer inhibitor such as raltegravir; tenofovir is less often used in the backbone of the tri-therapy). In the newborn should be used oral zidovudine (ZDV) for 4 to 6 weeks. If ART was initiated late during pregnancy or the mother received nevirapine (NVP) or raltegravir (RAL) during pregnancy or prior to delivery, the newborn should receive ZDV plus NVP or ZDV plus RAL. If pregnant is a woman who had received many treatments with resistance to some ARVs, it may be indicated triple therapy to the newborn ((ZDV+lamivudine+NVP or ZDV+lamivudine+RAL). With the application of HIV VT preventive ART and/or therapeutic of HIV infection, more other measures, it has been possible to reduce the VT of this virus to±1-2% in the US, Europe and many other countries, including Chile, with that in these places the emergence of new cases of HIV-infected children has been lower.1– 12,13,16–8,20–28
Unlike other countries, Chile has seen a sustained increase in HIV/AIDS cases, an increase that has grown in recent years. In the Figure 1 is shown the new cases of HIV, according sex, 2010-2018.2, 22–28 Exception HIV infection in children <13 years, which has decreased due to the progressive implementation of HIV VTPPs from 1995 and the dictation by the Ministry of Health (MINSAL) of the Norm of Prevention of Vertical Transmission of HIV in 2005.20,21 Thus the rate of HIV VT before 1995 (without VTPP) that was >35%, decreased between the years 1995-2005 (with VTPPs) to 2% in the binomials in VTPP of HIV, remaining until today in a figure <2%.2,22–30 In the Figure 2 is shown the evolution of HIV VT in binomials in VTPPs (Committee data).
HIV infection in children and adolescents in Chile
In Chile, in 1990 the Committee of Pediatric HIV/AIDS, was established by the Chilean Society of Pediatrics (SOCHIPE), with representatives from all regions of the country and its objectives the detection of children exposed to HIV (children of HIV-positive mothers) and children already infected with HIV, as well as the development of guidelines of study and management, furthermore the prevention and treatment of children. To this end, this Committee works in conjunction with the Department of Programs STD and HIV/AIDS, MINSAL, in the Pediatric HIV/AIDS Care Program.
HIV infection in children is defined as infection occurring in children under 13 years and this is primarily due to VT. Transmission from other causes such as sexual, drug addiction, tattoos, etc31,32 is added in the over>13s. The identification of the child/adolescent infected with HIV can be done by detecting pregnant women with HIV or detecting a child/adolescent already infected with HIV.33 In the case of a pregnant woman, she may be a known HIV woman who becomes pregnant or can be detected the HIV infection in the woman during pregnancy. Detection of HIV in pregnant women can be done because it has, a) risk factors (eg. prostitution, promiscuity, drug addiction, partner with risk factors, etc.), clinical manifestations suggestive of HIV (e.g. vulvar contagious molluscum) or, HIV-specific manifestations (e.g. hairy leukoplakia), or b) by requesting the test of HIV (remember HIV VT Prevention Norm 2005.20,21 The detection of HIV infection in the pregnant woman allows HIV VTPPs to be applied and thereby decrease the chance that the product of gestation will be born infected.16, 20,21
When a pregnant woman is HIV (+), she can also transmit other sexually transmitted diseases (STDs) such as syphilis, hepatitis B and C, etc., and that there is synergy between HIV and these STDs. In addition remember that some of these STDs are part of TORCH syndrome. One should think of HIV in infants with suspected TORCH Syndrome.33 The newborn of mothers HIV (+) are called Newborn Exposed to HIV because although born with HIV antibodies these antibodies can be passive antibodies transmitted by the mother or can be own antibodies because is infected. As passive antibodies can persist up to 18 months or more, the presence of HIV antibodies in the newborn not serve to certify that is infected. Own antibodies, of being infected, persist for life. So, to confirm the diagnosis of HIV in the newborn, the detection of antigens (Ag) of HIV or better of the nucleic acid of HIV, should be used. The latter may be performed by detecting the HIV DNA by the technique of polymerase chain reaction (HIV-DNA-PCR).16, 20,21,34
In managing the newborn exposed to HIV should avoid invasive monitoring. A gentle oropharyngeal aspiration should be done with a machine, bathing (chlorinating the washing water before removing it), and disinfecting the sites where injections are to be placed (e.g. vitamin K). Breastfeeding should be discontinued (with cavergoline or bromocriptine) and artificial lactation indicated (in Chile MINSAL provides formula up to 6 months of age, age at which other types of milk go free). It should begin VT preventive ART (ZDV, ZDV+NVP, ZDV+RAL as appropriate).20,21 As in Chile the BCG vaccine is given every newborn, in the case of a child exposed to HIV,35 to place the BCG vaccine must wait for the result of the CD4 count or percentage or, better, wait the result of the specific study of HIV, if the CD4 count or percentage is normal for age (>1500 CD4/mm3 or >35%) or the result of the first HIV-DNA-PCR is (-) can be vaccinated.20,21,36 The evaluation of the newborn must be with a complete physical examination (to detect if he or she already comes with some manifestation of HIV or TORCH), and with general laboratory tests (complete blood count, liver function, etc.), infectologic tests (cytomegalovirus, other STDs , etc.), and start the specific study of HIV, taking the first sample for antibodies, antigen and HIV-DNA-PCR before 48-72 hours of life. Must be notified immediately to MINSAL and send the newborn to the Pediatric HIV / AIDS Care Program of the Health Service or of the Region.20,21,33
In Chile, the HIV DNA-PCR test was implemented in 1992 and officially since 1997, which lowered the age of confirmation of HIV infection in children over 20 months (before 1992 confirmation was made by serology and in children infected antibodies persist beyond 18 months age when maternal antibodies have disappeared) at the age of 3 to 4 months today.33 Thus, for confirmation or discarding of HIV infection in newborns, is requested HIV-DNA-PCR. In Table 1 is shown the samples to be taken in the HIV exposed newborn to confirm or discard the infection.34 Depending on which samples result HIV (+) will determine whether the HIV infection was acquired in utero or intrapartum/peripartum. In Table 2 is shown that samples of HIV-DNA-PCR must result (+) to determine that the infection was acquired in utero or intrapartum or peripartum.37 The detection of the already infected child can be done on the basis of, family history (eg. relative HIV (+), risky behaviors in a family member, etc.), personal history (eg. risky behaviors, frequent or prolonged illnesses, transfusions, etc.), clinical manifestations at the time of consultation (eg. adenopathies, hepato-splenomegaly, thrush in a major infant , opportunistic infection, etc.), laboratory abnormalities (e.g. anemia with high VHS without another cause), request the HIV test.33
Samples |
|
|
|
|
|
|
|
|
|
Table 1 Samples for specific diasgnostic in HIV exposed newborn, son of HIV (+) mother
Utero acquired infection: |
HIV DNA PCR before 48-72 hours of life: |
-Positive result. |
-Confirmed with following tests. |
Intrapartum acquired infection or very close to delivery: |
HIV DNA PCR before 48-72 hours of life: |
-Negative result. |
-2nd sample (> 1 week of life): positive. |
-3th: positive results confirming 2nd sample. |
Whenever the newborn does not receive maternal or nurse breast feeding. |
Table 2 HIV infection acquisition period for vertical transmission
From a clinical point of view, pediatric HIV infection is characterized by a shorter incubation period than infection in the adult, and shorter in VT than in horizontal transmission, it presents with a wide spectrum of manifestations, from mild to severe (AIDS), which may be nonspecific, infectious, pulmonary, neurological and other. They are common childhood pathologies, but they occur more frequently or more severely, or longer lasting, or atypically, or more difficult to treat. This is a differential diagnosis of all common and unusual pediatric diseases.33 Table 3 lists samples to be taken to confirm or rule out HIV infection in children under 18 months of age. In the more than 18 months a confirmed serological result (+) is sufficient to confirm the infection.34 The management of these children by the Committee has been multidisciplinary, initiated from birth in exposed newborns and from the moment of their identification in children subsequently detected in life.33 Figure 3 shows the workflow diagram to follow in the Pediatric HIV/AIDS Care Program when an HIV-exposed newborn is detected or a child suspected of HIV is detected.
Age Children |
Confirmation Infection |
Discarding Infection |
Newborn |
At least two samples of the PCR (+) regardless first sample before 48 hours |
At least 2 samples with the PCR (-) excluding the first sample before 48 hours * |
Children ≥1 month and <18 months |
2 PCR should be (+) |
2 PCR should be (-) * |
Children ≥18 months |
Is enough 1 PCR (+) and / only Serology (+) |
Just one PCR (-) and / or Serology (-) |
*Although HIV antibodies are (+) (passive antibodies). |
||
PCR: HIV-DNA-PCR. |
||
Table 3 HIV infection in children: Laboratory Diagnostics (ISP; December 1997)
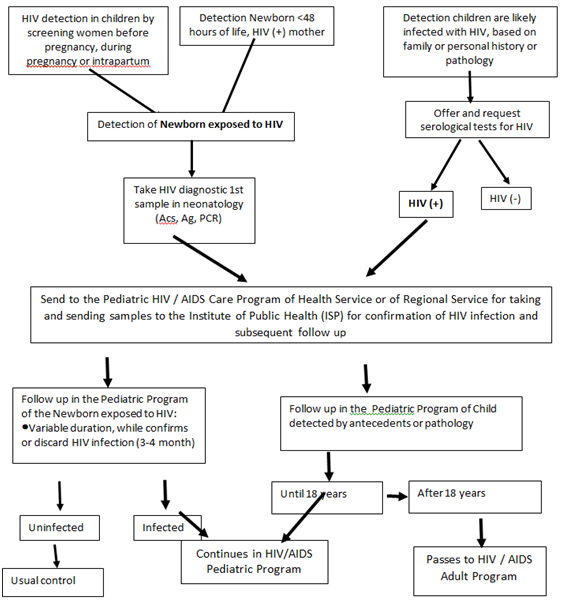
Figure 3 Flow chart Pediatric HIV / AIDS Care Program.
Source: Pediatric HIV / AIDS Committee, SOCHIPE.
Since the identification of the first HIV-infected child in Chile in 1987, to date there has been an improvement in the detection of HIV-infected children and in their access to ART; the detection has been increasingly precocious, identified at younger ages and with less clinical and immune commitment, which together with the indication of ART has resulted in better quality and prolongation of life and less lethality.29,30 In Chile, two Cohorts have been studied, the first from 1987 to 2008 and the second from 1987 to 2014. According to both Cohorts, most HIV-infected children acquired the infection on VT (95%).29
In relation to the identification of infection in children, according to the 1st Cohort, until 2008 the suspicion was mainly due to the pathology of the child (almost 50%) and less than a third (29%) by maternal history; since then, although the number of children detected by maternal history or entering HIV VTPPs has increased, children are still being identified belatedly by pathology (42% in the second cohort). According to the second Cohort, the detection on maternal basis has been 35% and by admission to the VTPP 10%.29
In Chile, with the application in some mother-son binomials of the original PACTG 076 protocol (with only ZDV), plus the other prevention measures already identified, a reduction in HIV VT was achieved from 30-35% in the period prior to 1995 to 9.5% at the end of 1997; its continuation in the following years and the incorporation into the pregnant of bi-therapy (ZDV+Lamivudina) and currently of tri-therapy antiretroviral, decreased VT to 2% between 1998 and July 2005, a figure that has remained so far in the binomials in which it has been applied. Between 1998 and July 2005, only 8 infected children were born of 401 mother-child binomials that received VT preventive measures (VT 2%),20,21,29 which contrasts with the 162 HIV children (+) who have been detected until the end of 2013 and who were born in the study period of mothers who were not subjected to any prevention measures (111 between 1998 and July 2005, study period , and 51 since that last date at the end of 2013).29 Traducción al Inglés.
Between 2006 and July 2014, years in which the HIV VT Prevention Norm already operated,20–21 were detected 127 HIV (+) children, half of whom were born in those years. In the Figure 4 is shown that with the application of Norm of VT Prevention from 2005, there was a decrease of detected cases, but no totally). Of these, in 59 the acquisition of the infection was on VT and of them half 29 did not receive HIV VTPPs and the other half 30 did. Of pregnant women without VTPP, it points out that half14 did not receive it because they had an HIV test (-) during pregnancy (until that date, pregnant women without risk factors were asked for a single HIV test at the first pregnancy check). Of those receiving VTPP in only one third was complete (antenatal, natal and in newborn ART, plus the other measures identified) for being diagnosed HIV (+) before or during pregnancy; in the rest it was incomplete (ART only in the period of childbirth and in the newborn or only in the newborn, with all or only some(s) of the other preventive measures), due to late diagnosis of HIV and /or various other causes such as regular or bad adhesion, drug addiction, failures in the health system (no HIV test offer, no result rescue, no VTPP offering, among the most frequent).29,30 All of this has meant that the transmission is maintained at±2% in those binomials with full or incomplete VT prevention.
The increasingly early clinical detection has allowed identify children infected with less clinical and immunological commitment, according to data from the first cohort 41% of children was detected in AIDS stage (CDC Classification 1994),32 which in the second cohort dropped to 27.8%. In the first cohort 59% was detected in NO AIDS stages which increased to 72% in the second cohort (stage N [17.8%], stage A [26%] and stage B [28.2%]). Most children detected by pathology were detected by nonspecific manifestations (27%) and infections (39%). Among infections present in this children, in the debut or during the course of the infection, the most common were by usual agents in children (eg. bacterial pneumonia, septicemia, meningitis) and by opportunistic agents, particularly Cytomegalovirus (CMV) and Candidas, and less frequent, by Pneumocystis jirovecii, Cryptosporidium, herpes simplex virus, Mycobacterium tuberculosis and Mycobacterium avium. Hematologic manifestations (15%), nutritional (11%) and neurological (spastic paraparesis, hypotonia, microcephaly) (8%) were present in fewer cases.29, 32
Improvement in preventive management (vaccines, prevention of opportunistic infections, etc.) and in the treatment of the consequences of HIV infection (e.g. treatment of anemia, treatment of habitual and opportunistic infections) and in the treatment of HIV infection (ART) has led to an increase in survival and decreased lethality, in Chile more than 70% of VT-infected children are now in adolescence and many have come under control with adult specialists.29,30,35,36–42 According to Cohort 2, of the total of 375 pediatric patients diagnosed by ISP from 1987 to 2014, 73 (19.6%) had died. Thirty-three cases (45%), died in the period prior to 2000, 25 cases (34%) from 2000 to 2005 and 15 (20.5%) from 2005 to 2014. In the first period the causes of death were mainly infectious (70%), by bacterial and opportunistic agents, especially CMV; several of those who died were detected already on AIDS in the first months of life or even post-mortem, most of them with CMV pneumonia; 100% of those who died had not received ART. In the second period, infections declined as a cause of death, matching opportunistic and bacterial; cancers appeared; 36% of those who died received ART. In the third period, a further decrease in infections was observed as a cause of death, especially from opportunistic infections, and cancers increased; 87% received ART.29
Of the first children identified by the Committee, several have already become adolescents and young adults. Several women have become pregnant and their children were born without HIV infection through the application of HIV VTPPs.29
None.
The author declares that he has no conflicts of interest.
None.

©2019 Wu. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.