International Journal of
eISSN: 2574-9889


Research Article Volume 4 Issue 3
1Department of Inmunobioquimica, Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes, Mexico
2Department of Ginecologia y Obstetricia, Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes, Mexico
3Department of Subdireccion de Servicios Auxiliares de Diagnostico, Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes, Mexico
4Department of Unidad de Materno-Fetal, Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes, Mexico
5Department of Salud Sexual y Reproductiva, Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes, Mexico
6Department of Biologia de la Reproduccion, Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes, Mexico
7Department of Fisiologia y Desarrollo Celular, Instituto Nacional de Perinatologia Isidro Espinosa de los Reyes, Mexico
Correspondence: Hector Flores-Herrera, Departamento de Inmunobioqumica, Instituto Nacional de Perinatologa Isidro Espinosa de los Reyes, Montes Urales 800, Colonia Lomas de Virreyes, CP 11000 Ciudad de Mexico, Mexico, Tel 15 2555 5209 705
Received: May 21, 2018 | Published: May 25, 2018
Citation: Mijangos MFV, Olvera-Valencia M, Pérez-Sánchez JE, et al. Secretion profiles of three extracellular matrix metalloproteases in severe preeclampsia. Int J Pregn & Chi Birth. 2018;4(3):151-155. DOI: 10.15406/ipcb.2018.04.00100
Background: The cellular and molecular factors that contribute to the development of preeclampsia (PE) have not been determined; however, the inadequate invasiveness and remodeling of the syncytiotrophoblast cells on the maternal spiral arteries has been considered the main axis leading to PE development. The process of invasiveness is finely orchestrated by the balance between the production of extracellular matrix metalloproteases and their tissue inhibitors MMP/TIMPs. The present study was conducted to evaluate the activity profile of proMMP-2, -3 and -9 in maternal serum of healthy pregnant patients with 34-weeks of gestation (n=38) and pregnant patients who admitted the Emergency Unit diagnosed with severe preeclampsia (n=50). In all cases blood samples (5mL) were taken from the peripheral vein. The maternal serum was obtained for the analysis of the MMPs by electrophoresis in gels of activity at 8% acrylamide/Bis with 5mg/mL of gelatin (MMP-2 and -9) or 15mg/mL casein (MMP-3) as substrate. The lysis bands were visualized in white light and their optical density was determined.
Results: The results indicated that in patients with PE significantly increased 1.3- (p=0.015), 1.9- (p=0.002), and 1.6-fold (p=0.007) the form proMMP-2, -9 and -3 respectively compared with healthy patients. In these same trials, we observed that the active form of MMP-2, -9 were 2.7- (p≤0.001) and 3.3-fold higher (p=0.035) with respect to healthy patients.
Conclusion: These results suggest that proMMP-2, -3 and -9 can be used as biomarkers in the diagnosis of PE.Keywords: preeclampsia, extracellular matrix metalloproteases, perinatology, proteinuria, glutamic pyruvic transaminases, glutamic oxaloacetic
Preeclampsia (PE) is a hypertensive disease that affects the normal development of pregnancy, its prevalence is approximately 8% of the total of pregnancies attended annually, of which 10% are patients with no risk factors, and up to 25% are patients with preexisting chronic hypertension1,2 and is associated with intrauterine growth restriction and neonatal complications. The reported prevalence in the National Institute of Perinatology was 10% in the last five years. PE is established from week 20 with the onset by first time in pregnancy hypertension and proteinuria; however, clinical data appearmore often after 28 week of gestation, during the third trimester, and when severe PE appear changes in glutamic oxaloacetic and glutamic pyruvic transaminases. Unfortunately the clinical signs and biochemical markers are not usually evident until PE has development, compromising maternal-fetal well-being.1
During the pregnancy development, the trophoblast migration and invasion through the decidua and maternal uterine spiral arteries events are crucial, during this process, invasive trophoblast replace vascular endothelial cells as the uterine arteries are remodeled from narrow, pulsatile and high resistance tubes to wide, non-pulsatile and low resistance vessels.2,3 These vascular changes occur by invasion and migration of the trophoblast in two stages producing modification in: 1) decidual segments of the spiral arterioles between 12-14 weeks and 2) myometrial segments between week 16 to 22. These modifications are produced by activity of the extracellular matrix metalloproteases (MMPs) secreted by the trophoblast cells.2,4−7
The PE is a result of por vascular response of the placentation, due to the inhibition of the second wave of trophoblastic migration, inducing endothelial damage, increased activation and platelet consumption, changes in the production and catabolism of prostaglandins in uteroplacental tissues and umbilical, decreased nitric oxide and increased endothelin causing decreased uterine-placental flow.2,3,5,8
Recently, the role of MMPs in the development of PE has been described, which is inadecuate degradation of the basement membrane proteins and prevent invasion of the extravillous trophoblast in an adequate manner.2,6,9 MMPs are zinc-dependent endopeptidases capable of degrading the components of the extracellular matrix and carry out different biological functions such as cell cycle regulation, connective tissue rupture, angiogenesis, organogenesis, scarring, inflammatory response, autoimmune diseases and carcinogenesis.3,10−12 The MMPs are divided into types according to the substrate they degrade: collagenolytics (MMP-1, -8, -13, y -18); gelatinolytics (MMP-2, -9); stromelysinoliytic (MMP-3, -10, -11), matrilysinolytic activity (MMP-7, -26) associated with MT-MMPs membrane (membrane-type matrix metalloproteases) and those that are not classified in the previous categories (MMP-12, -20, -22, -23, -28) and the a disintegrin and metalloproteinase (ADAM).3,4,7,9,11,12
A comparative study was conducted between the activity of proMMP-2, -3 and -9 in the serum of pregnant patients healthy (n =38) and pregnant patients with clinical evidence of severe PE (n=50) who had at the time of pathological development with 34-weeks of gestation. The hypothesis in this study was that in patients with severe PE would have a significant increase in the activity of proMMP -2, -3 and -9 with respect to healthy patients. The present study was approved by the research and ethics committees of Instituto Nacional de Perinatología (INPerIER; registration number 212250-3210091). In all cases informed consent was obtained.
Pregnant patients admitted to the Emergency Unit due to obstetrics complications. The clinical data of these patients were diagnosed as severe PE with 34-weeks of gestation (n=50) according to the American College of Obstetricians and Gynecologists (ACOG, 2013) guidelines. PE patients were included in the study previous of the therapeutic treatment. Healthy pregnant patients were included in this study with a single pregnancy and without obstetric complications with 34-weeks of gestation (n=38). The blood samples of all patients were taken from the peripheral vein, in only occasion and collected in tubes BD Vacutainer with K2-EDTA (Becton-Dickinson, USA). The serum was obtained from 5 milliliters of maternal blood samples and stored at -80°C until assayed the proMMP-2, -3 and -9 activity. Total protein content in serum samples was determined by the Bradford (19076) methods using bovine albumin as standard.13
Determination of metalloprotease by zymography gel activityThe secretion of extracellular matrix metalloproteases from plasma was determined by activity gels assay as reported by our research group.14 SDS-polycrilamide gels were co-polymerized whit gelatin 1mg/mL (proMMP-2 and -9) or casein 6mg/mL (proMMP-3) and loaded using 0.75µg of total protein in non-denaturing buffer. As standard of activity for proMMP-2 and -9 we used medium from U937 promyelocytic cells (ATCC Manassas, VA, USA). The samples were diluted with sample buffer (0.5M Tris; pH. 6.8), 10% glycerol. 1% bromophenol blue). After electrophoresis at a constant voltage of 10mA at 4°C, SDS was retired by incubation with 1% Triton X-100 (twice) for 15min at room temperature under constant agitation. The gels were incubated overnight at 37°C in activation buffer (50mM Tris pH7.4, 0.1M CaCl2, 0.15M NaCl, and 0.2mg/mL NaN3). The gels were stained for 60 minutes with 0.2% Coomassie brillant blue R-250 (Sigma-Aldrich, St Louis, MO, USA) in methanol:acetic acid:glicerol:water (10:10:10:70) at room temperature and distained in a solution containing methanol:acetic acid:water (10:10:80) until intense bands were seen. The activity of proMMP-2, -3 and -9 was estimated by densitometry using the EpiChemi Darkroom gel documentation system (UVP; CA, USA) and was expressed in relative densitometry units.
Statistical analysisThe activity of proMMP-2, -3 and -9 between the group of preeclampsia with respect to the control group was carried out by means of the ANOVA test followed by the post-hoc analysis of Mann-Whitney (Sigma Stat, v10, USA). The data were shown as the mean±standard deviation (SD) and a statistically significant difference of less than 0.05 will be considered.
The maternal characteristics of the patients in this study are described in Table 1. In the cases of PE no significant difference were detected in maternal age, BMI, uric acid, and creatinine concentration with respect to healthy pregnant patients with 34-weeks of gestation (Table 1); however, systolic (p<0.001) and diastolic (p<0.001) blood pressure, and cesarean delivery (p=0.048) showed statistically significant differences. Finally, the values of hepatic function of LDG, GOT, and GPT increased in patients with PE development (Table 1).
In the newborns of mother with severe PE a significant difference was found in gestational weeks 35.8±3.6 (p=0.007; Table 2), weight 2,297±785.7 (p<0.001), and resolution by cesarean 85.1% (p=0.048) with respect to newborns of healthy pregnant. No difference was detected in newborn sex (Table 2).
Parameters |
Severe preeclampsia (n=50) |
Healthy pregnant patients (n=38) |
p |
Age (year) |
30.2±8.0 |
29.3±9.2 |
0.642 |
BMI (kg/m2) |
31.0±4.7 |
25.1±2.3 |
< 0.001 |
Systolic blood pressure (mm Hg) |
154.2±16.1 |
104.2±8.3 |
< 0.001 |
Diastolic blood pressure (mm Hg) |
95.9±8.5 |
67.5±8.0 |
|
Deliver vaginal/cesarean (%) |
14.9/85.1 |
33.3/66.7 |
0.048 |
Hematic biometrics |
|||
Maternal hemoglobin (%) |
12.3 (1.2) |
12.3 (1.4) |
0.953 |
Platelets (103/mm3) |
209.0 (49.5) |
200.9 (70.6) |
0.541 |
Leukocytes (109/L) |
8.6 (1.8) |
9.6 (3.5) |
0.124 |
Biochemical parameters |
|||
Uric acid (mg/mL) |
5.2±1.2 |
5.3±1.1 |
0.784 |
Creatinine (mg/mL) |
0.6±0.2 |
0.6±0.2 |
0.627 |
LDG (UI/L) |
317.5±187.2 |
ND |
|
GOT (UI/ml) |
33.0±42.6 |
ND |
|
GPT (UI/mL) |
31.7±7.0 |
ND |
|
Table 1 Characteristics of the study population and concentration of biochemical parameters
BMI, body-mass index; LDH, lactate dehydrogenase; GOT, glutamic oxaliacetic transaminases; GPT, glutamic piruvic transaminases; ND, no determined; data expresses as mean ±SD; statistical difference (p<0.05)
Severe preeclampsia (n=50) |
Healthy pregnant patients (n=38) |
p |
|
Gestation age at birth (weeks) |
35.8 ± 3.6 |
37.7 ± 2.7 |
0.007 |
Weight (gr) |
2,297 ± 785.7 |
3,065 ± 453,0 |
<0.001 |
Sex n(%) |
Male 32 (68,1) |
Male 18 (50) |
0.095 |
Female 15 (31,9) |
Female 18 (50) |
||
Birth resolution n(%) |
In labor 7 (14,9) |
In labor 12 (33,3) |
0.048 |
Cesarean 40 (85,1) |
Cesarean 24 (66,7) |
||
Tabla 2 Characteristics of the newborn
Data expresses as mean ±SD. Statistical difference (p<0.05)
The determination of the proteolytic activity of proMMP-2 and -9 (gelatin) and proMMP-3 (casein) were carried out by electrophoresis assay with specific substrates.
Gelatinolytic activity: Figure 1 shows the secretion of proMMP-2 and its active form in healthy patients (Figure 1A) and in patients with severe PE (Figure 1B). The severe PE significantly increased 1.3-fold the secretion of proMMP-2 compared to healthy patients (p=0.015; Figure 1C). In these same trials, we observed in patients with PE development the active form of MMP-2 (actMMP-2) which increased significantly 2.7-fold with respect to healthy patients (p≤0.001; Figure 1D). In patients with severe PE the relationship between the act/pro MMP2 increased 2-fold with respect to healthy pregnant patients.Figure 2 shows the secretion of proMMP-9 and its active form in healthy patients (Figure 2A) and from patients with preeclampsia (Figure 2B). The development of PE significantly increased the secretion of proMMP-9 1.9-fold over healthy patients (p=0.002; Figure 2C). In these same trials we observed in patients with PE the secretion of active form of MMP-9 (actMMP-9) which increased significantly 3.3-fold with respect to healthy patients (p=0.035; Figure 2D). In patients with severe PE the relationship between the act/pro MMP9 increased 1.78-fold with respect to healthy pregnant patients.
Caseinolytic activity: Figure 3 shows the secretion of proMMP-2 and its active form from healthy patients (Figure 3A) and from patients with preeclampsia (Figure 3B). The development of severe PE significantly increased the secretion of proMMP-3 by 1.6-fold compared to healthy patients (p=0.007; Figure 3C). In these same trials we observed in the patients with PE the secretion small forms of actMMP-3; however, we were not able to make the quantitative due to its optical density.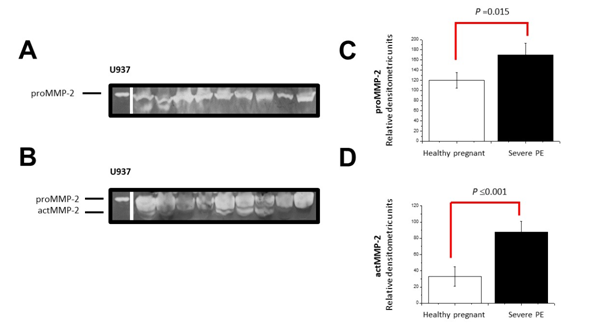
Figure 1 Differential secretion of proMMP-2. The activity of proMMP-2 obtained from the serum of healthy patients (A) and with clinical development of PE (B) was performed on gelatin gels as a specific substrate. The gel is a representative image of all the samples. The semiquantitative analysis was determined by optical density and the proMMP-2 (C) and actMMP-2 (D) form was compared between healthy patients and PE patients. The data is presented as the mean±SD. In each case, statistical significance is presented by the Mann-Whithney test.
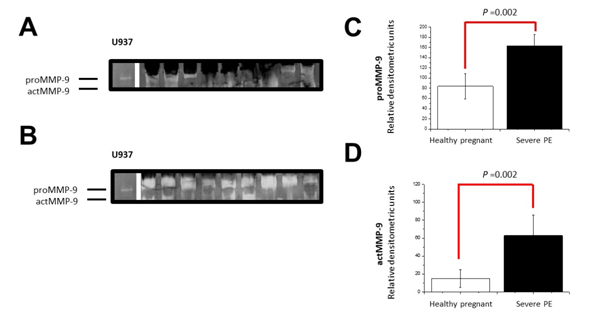
Figure 2 Differential secretion of proMMP-9. The activity of proMMP-9 obtained from the serum of healthy patients (A) and with clinical development of PE (B) was performed on gelatin gels as a specific substrate. The gel is a representative image of all the samples. The semiquantitative analysis was determined by optical density and the proMMP-9 (C) and actMMP-9 (D) form was compared between healthy patients and PE patients. The data is presented as the mean±SD. In each case, statistical significance is presented by the Mann-Whithney test.
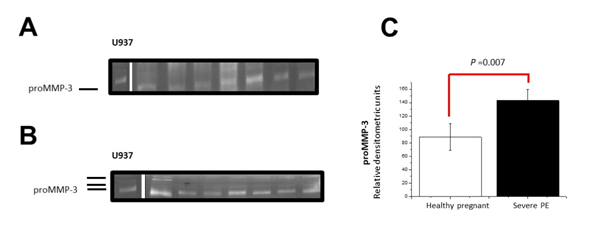
Figure 3 Differential secretion of proMMP-3. The activity of proMMP-3 obtained from the serum of healthy patients (A) and with clinical development of PE (B) was carried out on gels with casein as a specific substrate. The gel is a representative image of all the samples. The semiquantitative analysis was determined by optical density and the proMMP-3 form was compared between healthy patients and those of PE (C). The data are presented as the mean±SD. In each case, statistical significance is presented by the Mann-Whithney test.
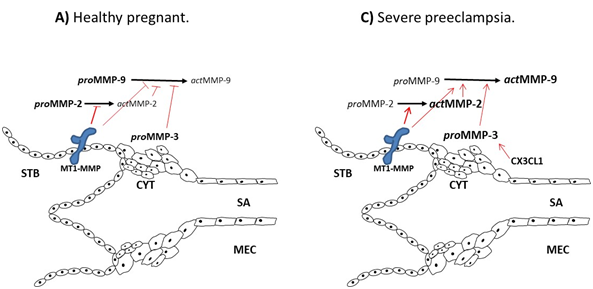
Figure 4 Action model for the activation of MMPs. During pregnancy are mainly proMMP isoforms which are inactive as their binding site to substrates is not available. A portion of the proMMP can be found actively (A); however, they are inhibited by tissue inhibitors of MMPs (TIMP). In the establishment of preeclampsia membrane-type metalloprotease (MT-MMP) induces the activation of proMMP-2 and -9 by removing the propeptides near the catalytic site increasing the production of the actMMP (B) forms. The inflammatory and chemotactic environment induces the activation of MMP-3 which increases the production of MMP-9. Membrane-type MMP (MT1-MMP), syncytiotrophoblast (STB), cytotrophoblast (CYT), spiral arterial (SA), maternal endothelial cells (MEC).
The endometrial invasiveness and remodeling of the maternal spiral arteries by the syncytiotrophoblast cells depends on a regulation that is established with maternal endometrial cells.15−17 Deficiencies in this cellular communication leads to the development of preeclampsia. One of the mechanisms in endothelial damage is the massive secretion of the MMPs which have been associated in the development of the PE.6,8
The main findings in the present study are 1) the proMMPs -2, -3 and -9 increased in plasma of patients with severe PE, and 2) the actMMPs -2, -3 and -9 forms were observed in the patients with severe PE. It has been shown in patients with PE induced MMPs production is associated with endothelial dysfunction as a result of inadequate cell invasiveness,3,7 as well as different inflammatory cytokines (IL-1β, TNFα), and chemokines production.18,19 Our results indicate that the ratio of the act/proMMP isoforms in patients with severe PE is found mainly at actMMP with respect to healthy pregnant patients. This suggests that the regulatory system of MMPs is modified in the endothelial microenvironment of patients with severe PE.
It has been shown that MMP-2, and -9 are associated with the pathological development of PE;3,5 however, there are controversies indicating a decrease in the MMP-9 form, and -3 in patients with PE.3,11 Probably the difference could be due to the type of MMP that are being identified. Our results show the band of active forms for both MMP-2 and -9.
MMP-3 has a broad spectrum on extracellular matrix substrates which include fibronectin, laminin, vitronectin and different collagen than other MMP-3 such as MMP-2 and MMP-9 which have a proteolytic activity activated by particular enzymes. MMP-3 plays a very important role on the remodeling process and that an abnormal expression of this MMPs has been associated to different pathologies. Our results indicate that PE significantly increases the production of MMP-3 (Figure 3C).
Figure 4 shows a model of interaction between MMPs in both healthy pregnant patients and in the development of preeclampsia. During pregnancy, the proMMP-2 and -9 forms are in their zymogen state; however, a minimal production is transformed to its active forms (Figure 4) being regulated by the production of its specific tissue inhibitors (TIMPs)20,21 as well as by the MT1-MMP.22 In the establishment of preeclampsia, the regulatory factors are modified so that the MT1-MMP removes the propeptide associated with the active site of proMMP, inducing a conformational change towards its actMMP form.17,23,24
Finally, these results allow in another stage of the study to demonstrate the variations in the expression of tissue inhibitors TIMPs which will allow understanding the mechanism of activation of MMPs. These findings, such as those recently published by Feng et al.25 suggest that the active forms of MMP-2, and -9 could serve as biomarkers in patients with preeclampsia.25
The pathological mechanism of preeclampsia induces a deregulation on the mechanisms of inhibition on the MMPs promoting an increase in the secretion of its active forms mainly of the actMMP-2 and -9.
This study was supported by Grant 212250-3210091 (to HFH) from Public Health of Instituto Nacional de Perinatología “Isidro Espinosa de los Reyes”, México. We thanks to Impulso a la Investigación Científica for the grant of to MFVM.
The authors declare that they have no competing interests.

©2018 Mijangos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.