International Journal of
eISSN: 2470-9980


Research Article Volume 3 Issue 1
1Public Health Institute of Belgrade, Serbia
2Institute of Virology, Serbia
3National Institute for Health and Welfare (THL), Finland
4University of Belgrade, Serbia
Correspondence: Tatjana Pljesa, Public Health Institute of Belgrade, Belgrade, Serbia, Tel +381642777386
Received: July 08, 2016 | Published: November 18, 2016
Citation: Pljesa T, He Q, Dakic G, Vignjevic-Krastavcevic M, Cirkovic I (2016) Polymorphism in Bordetella Pertussis Virulence Factors in Serbia before Switch from Whole Cell to a Cellular Pertussis Vaccine. Int J Vaccines Vaccin 3(1): 00058. DOI: 10.15406/ijvv.2016.03.00058
Background: Mass vaccination has significantly reduced the incidence of pertussis; however, the disease is re-emerging even in some countries with high vaccination coverage. In Serbia, whole cell pertussis vaccine was introduced in 1957 and it has been used since 2014.
Methods: To monitor changes in bacterial population, 77 isolates collected from 1953 to 2013 were studied. The methods included serotyping of fimbriae (Fim), genotyping of pertactin (prn), pertussis toxin S1 subunit (ptxA) and pulsed-field gel electrophoresis analysis (PFGE).
Results: Shift from ptxA2 to ptxA1 has been observed in isolates since the late of 1960s. In the period 1961-1979 genotype ptxA1 became as common as genotype ptxA2. After that, during the period of 1980-1989, the predominant ptx genotype was ptxA1. Re-appearance of the ptxA2 allele followed an addition of the two strains harboring ptxA1 in the vaccine in 1985. The allele prn1 was predominant among the Serbian isolates, though prn3 and prn11 have been detected since 1981. The prn2 allele was only found in one strain isolated in 1984, two of the four strains isolated in 2000 and three strains from 2011. Serotype Fim2.3 disappeared before 1980 and serotype Fim2 became predominant since then. The four vaccine strains represented four PFGE profiles. Twenty-two (40%) isolates tested by PFGE produced 22 distinct profiles. Twenty-four (43%) isolates had unique Serbian profiles (BpSBR).
Conclusion: The results of this present study indicate that the B. pertussis population in Serbia is different from other vaccinated populations and that this difference may be related to the vaccine used for 57years.
Keywords: bordetella pertussis, pertussis toxin, pertactin, pfge, serotyping, fimbriae, electrophoresis
Fim, fimbriae; prn, pertactin; PFGE, pulsed-field gel electrophoresis analysis; DTPw, diphtheria-tetanus-whole cell pertussis; ptxA, pertussis toxin subunit
Pertussis (whooping cough) is a worldwide infectious disease caused by the bacteria Bordetella pertussis.1 Despite the fact that the introduction of vaccination in the 1950s and 1960s has reduced pertussis morbidity and mortality, this disease is still prevalent2 and pertussis is still one of the leading causes of vaccine preventable deaths in the world.3 Pertussis is a respiratory tract infection transmitted by aerial droplets with an incubation period of 7-10days, which remains contagious for up to 3weeks after the appearance of the first signs if no treatment is given. Mass vaccination has significantly reduced the incidence of pertussis; however, the disease is re-emerging even in some countries with high vaccination coverage. The resurgence of pertussis in countries, such as the Netherlands, the United States, Canada and Australia, has been studied to find an explanation for its re-emergence.2,4‒7 Moreover, in these countries antigenic divergence with respect to pertussis toxin (Ptx) and pertactin (Prn) has been found between Bordetella pertussis vaccine strains and clinical isolates. In Serbia vaccination against pertussis has been from 1957. The diphtheria-tetanus-whole cell pertussis (DTPw) vaccine has been manufactured in the Institute of Virology, Vaccine and Sera Torlak, Belgrade, Serbia. The DTPw vaccine was given at 2, 4, 6, and 12months of age. A second booster dose with the mono pertussis vaccine was given at 4years of age during the periods from 1970 to 1981 and from 1990 to 2000. Last composition of the whole-cell vaccine has been used since 1985 and contained four B. pertussis strains. The vaccine strains were chosen in compliance with serotype, immunogenicity and specific toxicity. The four strains represented three serotypes: Fim2 (8/84), Fim2,3 (1772/57 and 2047/57) and Fim3 (23/81). The vaccine strains 2047/57 and 1772/57 represented ptxA2/prn1 genotypes, whereas the vaccine strains 23/81 and 8/84 harbor ptxA1/prn1 and ptxA1/prn2. All vaccines strains were in equal amount in the vaccine composition.8 The reported vaccination coverage in Serbia ranged from 79% to 98% (median, 90%) in 1981-2013.3 In Serbia, the whole-cell pertussis vaccine has been replaced with a cellular vaccine in 2014. The aim of this study was to analyze B. pertussis isolates circulating between 1953 and 2013 in Serbia by standard typing methods9 and to compare them with those circulating in other European countries, USA and Australia.
This study included 77 B. pertussis isolates. Detailed information on each of vaccine strains and clinical isolates was included in the Supplementary data. Clinical isolates were selected from the B. pertussis strain collection of the Institute of Virology, Vaccine and Sera Torlak, Belgrade, Serbia. Information on age, gender and vaccination was available in 64 patients. Their age ranged from 2months to 33years (mean, 6.06years; median, 5years). Bacteria were grown at 36°C for 72h on Bordet Gengou agar supplemented with 30% defibrinated sheep blood and subcultured on the same medium for 24h.10
Fimbriae serotyping of B. pertussis isolates
Serotyping was performed with monoclonal antibodies against Fim2 and Fim3 by slide agglutination test.9,11
Genotyping of Ptx S1 subunit (ptxA) and prn
The standardized genotyping of Ptx S1 subunit (ptxA) and prn was perform by sequencing and Light Cycler PCR.11‒13 These methods have been recommended for the epidemiological typing of B. pertussis isolates.9
PFGE analysis
For the PFGE analysis, six international reference strains were included9 and nomenclature was based on the defined profiles already observed in Finland (BpFINR) and Sweden (BpSR).11,12 Profiles assigned BpSBR have been found only among the Serbian isolates analyzed.
Statistical analysis
A chi-square test was used to compare frequencies of strain genotypes and serotypes between four time-periods (1953-1960, 1961-1979, 1980-1989 and 1990-2013). A p-value ≤0.05 was considered as statistically significant. Selection of time-periods was performed according to the epidemiological data.
Epidemiology of pertussis in Serbia
In Serbia, pertussis is a notifiable infectious disease, which is collected in the Infectious Disease Register of the National Public Health Institute. The incidence of pertussis in Serbia has been decreasing since the introduction of vaccination at the end of the 1950s and beginning of the 1960s (Figure 1). All isolates prior to 1960 were recovered from unvaccinated patients. In the period 1980-1989, 82.35 % of patients were vaccinated while there were 64.3% of vaccinated patients in the period 1990-2013. There were no epidemiological data for strains isolated from patients in period 1960-1979.
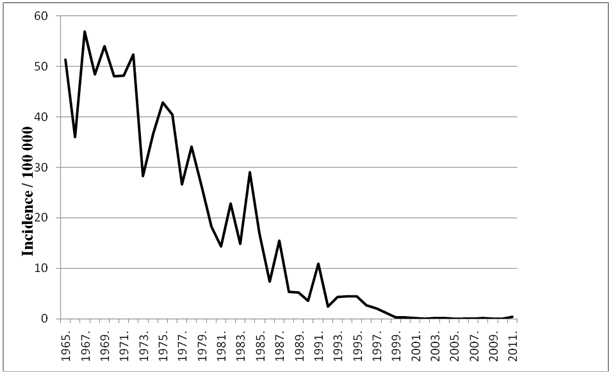
Figure 1 The incidence (cases per 100,000) of reported pertussis cases in Serbia between 1965 and 2011. Source: Infectious Disease Register, Institute of Public Health of Serbia “Dr Milan Jovanovic Batut”.
Fimbriae serotyping
All three serotypes, Fim2, Fim2.3, and Fim3, were observed among the clinical isolates. The frequency of each serotype has changed over time. Before the introduction of vaccination, the prevalent serotypes were Fim 2(38%) and Fim 2.3(62%). After the introduction of vaccination, the frequency of serotype Fim2.3 decreased, being significantly lower, 0% in 1980-1989 and 23% in 1990-2013, compared to those observed in 1953-1960and 1961-1979 (P = 0.00013 and 0.0003) (Table 1). Although the serotype Fim3 started to appear, Fim2 has been the most prevalent serotype during the study period (Figure 2).
|
Year of Isolation |
No of Isolates |
ptxA (no) |
prn (no) |
serotype (no) |
||||||
|
ptxA1 |
ptxA2 |
prn1 |
prn2 |
prn3 |
prn11 |
Fim2 |
Fim2,3 |
Fim3 |
||
|
1953-1960 |
21 |
0 |
21 |
21 |
0 |
0 |
0 |
8 |
13 |
0 |
|
1961-1979 |
9 |
4 |
5 |
9 |
0 |
0 |
0 |
0 |
6 |
3 |
|
1980-1989 |
34 |
31 |
3 |
27 |
1 |
3 |
3 |
22 |
0 |
12 |
|
1990-2014 |
13 |
10 |
3 |
4 |
5 |
1 |
3 |
7 |
3 |
3 |
|
Total |
77 |
45 |
32 |
61 |
6 |
4 |
6 |
37 |
22 |
18 |
Table 1 Temporal trends in serotypes and genotypesof pertussis toxin and pertactin in Serbia
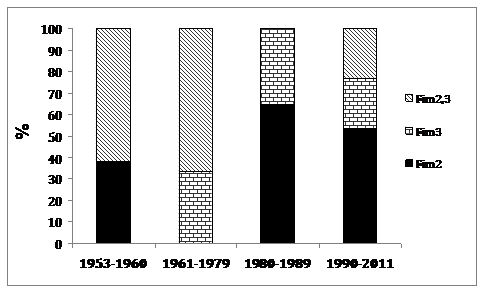
Figure 2 The frequency of Fim2, Fim2.3, and Fim3 serotypes of B. pertussis isolates circulating in Serbia.
Genotypes of ptxA and prn
All strains isolated from 1953 to 1960 were ptxA2 genotype. Shift from ptxA2 to ptxA1 has been observed in isolates since the late of 1960s. In the period 1961-1979 genotype ptxA1 became as common as genotype ptxA2. After that, during the period of 1980-1989, the predominant ptx genotype was ptxA1 (91,2%). Re-appearance of the isolates containing ptxA2 was noticed after the two strains harboring ptxA1 were added into the vaccine in 1985.8 During the period of 1990-2013, both ptxA genotypes were present in the population (Figure 3).
In the first two observed periods, 1953-1960 and 1960-1979, all isolated strains were prn1 genotype. Although the allele prn1 was mostly predominant among the Serbian isolates analyzed, prn2, prn3 and prn11occurred in some isolates since 1980s and finally prn2 genotype became predominant in the period 1990-2011. The allele prn3 and prn11 were first detected in 1981 and1984, respectively and became more frequent in 1990-2013. The prn2 allele was only found in one strain isolated in 1984, two of the four strains isolated in 2000 and three strains from 2011. The frequency of prn1 in 1953-1960 and 1961-1979 was significantly higher than those observed in 1980-1989 and 1990-2013 (P <0.001 in both groups) (Table 1 & Figure 4). The frequency of prn11 in 1980-1989 and 1990-2013 was significantly higher than that found in 1953-1960 and 1961-1979 (P <0.001 in both groups).
All the four isolates harboring prn3 contained ptxA1, whereas five of the six isolates harboring prn11 represented ptxA2. All the six isolates harboring prn11 were serotype Fim2, whereas three of the four isolates harboring prn3 were serotype Fim3.
PFGE profiles
The four vaccine strains represented four PFGE profiles (Figure 5). The 56 isolates tested by PFGE produced 22 distinct profiles (Figure 6). The five common profiles represented about two thirds of isolates (17 isolates with BpSR23, eight with BpFINR1, five with BpFINR9, four with BpSBR6 and three with BpSBR5). Twenty-four (43%) isolates had unique Serbian profiles (BpSBR). Change in PFGE profiles was observed over time. All PFGE profiles, except BpSR23, observed in 1950s disappeared since then. The profile BpSR23 was found in all the study periods. Of the 56 isolates tested by PFGE, 53 (95%) belonged to two clusters, having a high similarity with a minimum of 78% overall relatedness (Figure 6). Of the six isolates harboring prn11, four had the profile BpSBR6, one had BpSBR7, and one had BpSBR14. They all fell into the same cluster. Of the four isolates harboring prn3, two had the profile BpSBR8, one had BpSBR12, and one had BpSR127. They all fell into another cluster. Two isolates with prn2 had the profiles BpSBR9 and BpSR11. They fell into the same cluster as the isolates with prn3.
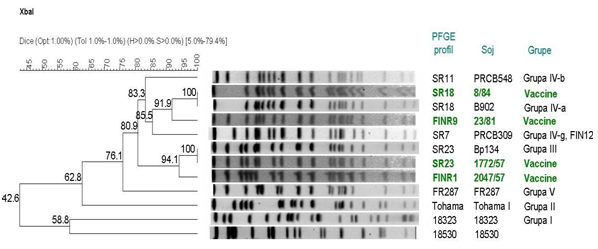
Figure 5 Dendrogram analysis of PFGE profiles of B. pertussis Serbian vaccine strains and their relatedness to isolates groups. The unweighted pair group method using arithmetic averages (UPGMA) with 1% band tolerance and 1% optimisation settings was used as the clustering method.
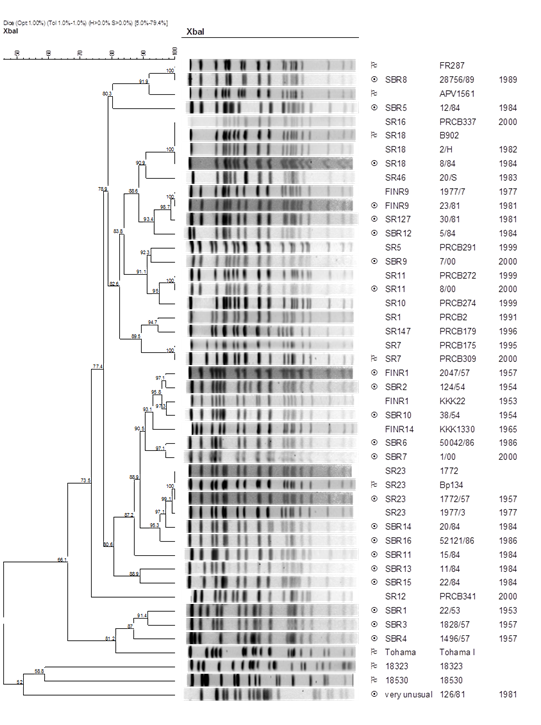
Figure 6 Dendrogram analysis of 22 PFGE profiles of B. pertussis isolates circulating in Serbia during 1953–2014. The unweighted pair group method using arithmetic averages (UPGMA) with 1% band tolerance and 1% optimisation settings was used as the clustering method. The symbol indicates international reference strains and Serbian vaccine strains. Of the isolates with identical profiles, only first one is shown.
Vaccinations with Pw vaccines were introduced in the 1940sto 1960s and have successfully reduced morbidity and mortality of pertussis throughout the world.14 Pw vaccines have been produced by different manufacturers, and the B. pertussis vaccine strains vary. The vaccine strains, both for whole-cell and cellular vaccines were usually isolated in the 1940s to1960s, and in many countries the vaccinations have selected circulating isolates dissimilar to the vaccine strains.4,12,15,16 Further, resurgence of pertussis has been observed in countries with long term pertussis vaccination.7,16‒19 In Serbia Pw vaccine has been in use from 1957 to 2014, and was manufactured in the Institute of Virology, Vaccines and Sera Torlak, Belgrade. In contrast to the many other countries, the Serbian vaccine contained four strains: two isolated in 1950s and two in 1980s. Of the four strains, one strain represented prn2 genotype and the other three prn1 genotype. The vaccine has remained unchanged since 1985, when the two newly isolated strains have been added to the vaccine composition. One of the added strains was strain 8/84 with prn2 allele.8 This unique formulation of the Serbian Pw vaccine provided us an opportunity to study possible effect of the inclusion of “contemporary” strains in the vaccine on temporal trends in B. pertussis population. The finding that frequency of the Serbian isolates representing prn2 was low and its appearance was late is striking. Polymorphism in Prn is essentially limited to region 1 and is located adjacent to an RDG motif implicated in adhesion.20 So far, 13 prn alleles have been identified.21 In many countries the allele prn1or prn7 is present in most vaccine strains and predominated in prevaccine era.14 However, the “vaccine type” strains were gradually replaced by “non-vaccine type” strains mainly prn2 after the introduction of vaccination. The prn2 is by far the most prevalent type in modern isolates.4,11,12,16,22‒24 Depending on the time when the pertussis vaccination was started and the potency of vaccine used, in most countries isolates containing prn2 were first observed in the 1970s to 1980s.4,9,11,12,23,25 In line with these observations, in Serbia one isolate (8/84) harboring prn2 was detected in 1984. The isolate was added in vaccine composition in 1985. After that, all isolates were prn1 or prn11 until 2000 when two out of four isolates contained the prn2 allele. The low frequency of prn2 strains and their relatively late emergence in Serbia may be due to the fact that the vaccine contained an isolate having prn2 allele. In this present study, four different prn alleles (prn1, prn2, prn3and prn11) were detected among the Serbian isolates. The alleles prn1 to prn3 have been observed in many countries.4,12,22-24,26 The allele prn11 was only reported in a recent study carried out in Australia, where all 5 strains containing prn11 were isolated in the same year (1982) and in the same region.26 In our study, however, the 6 prn11 isolates were detected in different years from 1984 to 2000. Of the six isolates, four had identical serotype and ptxA genotype. The difference between Prn1 and Prn11 was only one repeat in region 1: Prn1 has five repeats, whereas Prn11 has six repeats. These observations that most of the isolates from 1980 to 2013 contained the prn1 or prn11 allele suggested that the strains prevalent in the pre-vaccine era are still circulating in Serbia. The Serbian vaccine strains do not contain the allele prn3. However, strains harboring the allele prn3 were not isolated in the study at a frequency comparable to that seen in other countries.12,22,24,26 The exact reasons for the difference are not known. In addition to the vaccine composition, many factors such as immunity, density and dynamics of population can contribute to selection of the circulating strains. So far 8 ptxA alleles have been reported.21 In most countries, the allele ptxA2 and/or ptxA3 are present in most vaccine strains and predominated in the isolates circulating in pre-vaccine era.4,12,16,23 However, the “vaccine type” strains were gradually replaced by “non-vaccine” type ptxA1 after the vaccination was introduced. Our result was in agreement with the earlier studies. Shift from ptxA2 to ptxA1 was observed in isolates since the late of1960s, and predominant ptxA genotype in the period 1980-1989 was ptxA1. Interestingly, re-appearance of the ptxA2 allele followed an addition of the two strains harboring ptxA1 in the vaccine composition in 1985. After that moment, there were more ptxA2 isolates than previously. The high frequency of strains harboring ptxA2 in 1990-2013 was not comparable to that noticed in many other countries.4,12,23 Several studies have shown that Fim2 isolates predominate in unvaccinated population, while they are largely displaced by Fim3strains when vaccination is introduced with a Pw vaccine containing both Fim2 and Fim3.27,28 Although the vaccination has been used in Serbia since 1957, nearly half of the isolates studied from 1957 to 2013 were serotype Fim2. Increases in the incidence of pertussis have been reported in many countries with long vaccination history. Moreover, in many of these countries divergence between vaccine strains and circulating isolates have been found. Interestingly, the incidence of pertussis has been decreasing in Serbia during observed period. It is known that in vaccinated populations, symptoms of pertussis can be mild and the patients do not usually seek for medical help. Therefore, the possibility that incidence of pertussis is underestimated in this country cannot be excluded. Whether the low incidence of pertussis in this country is related to the vaccine used remains to be illustrated. King et al.,29 were the first to show that variation in Prn affects vaccine efficacy in the mouse model.29 It has been recently shown that the adequate bacterial elimination rates were observed in mice immunized and challenged with the same vaccine type strain30 and that the vaccine prepared from a recent isolate provided the highest mouse protection when compared to those prepared from the old isolates such as the strain Tohama I.31 The question of whether prn2 strains eventually became predominant in this country remains to be shown in further investigations. According to the observed findings, B. pertussis population in Serbia during observed period was different from other vaccinated populations and this difference may be related to the vaccine used. The effects of cellular vaccines on the circulating B. pertussis strains should be closely monitored. This study lays a good background for further monitoring of the circulating B. pertussis isolates in Serbia. The exceptionally stable vaccination history with a high vaccination coverage rate makes Serbia a good location for monitoring of the changes in the B. pertussis population after the introduction of a new vaccination program with cellular pertussis vaccines in 2014.
None.
Author declares there are no conflicts of interest.
None.

©2016 Pljesa, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.