International Journal of
eISSN: 2574-8084


Research Article Volume 6 Issue 1
1Department of Radiology, Special VM Medicalpark Kocaeli Hospital, Turkey
2Department of Medical Oncology, Bahcesehir University, Turkey
Correspondence: Kocaeli Hospital, Radiology Department 41140, Basiskele, Kocaeli, Turkey, Tel +90 262 888 30 00, Fax +90 262 888 39 00
Received: December 31, 2018 | Published: January 17, 2019
Citation: Yirgin IK, Koca D. Diffusion-weighted magnetic resonance imaging (DW-MRI) in suspected liver metastasis. Int J Radiol Radiat Ther. 2019;6(1):11-15. DOI: 10.15406/ijrrt.2019.06.00204
Background: We aimed to investigate suspected liver metastasis in cancer patients using diffusion-weighted magnetic resonance imaging (DW-MRI).
Materials and methods: In our oncology clinic between March 2016 and May 2018, we reviewed patients with suspected liver metastasis. All patients were previously assessed using abdominal ultrasonography, upper abdomen computerized tomography, upper abdomen magnetic resonance imaging, and/or positron emission tomography-computerized tomography. We performed DW MR imaging on all cases to determine the presence of metastasis.
Results: A total of 138 cancer patients were evaluated. Median age was 60 years (range: 17–85). Of these, 55.8%(77) patients were female, and 20.3%(28) patients had breast cancer. All patients (100%) had an upper abdomen computerized tomography scan. PET-CT was performed in 98(71.0%) patients. Upper abdomen magnetic resonance imaging was performed in 29(21.0%) patients. Twenty-four (17.3%) patients had upper abdominal ultrasonography. All patients had remaining suspicion of liver metastasis. For this reason, all patients (100%) underwent DW-MRI. Signs of malignancy were detected in 46(33%) of the patients using DW-MRI whose not detected by all other tests. The number of patients with newly detected hepatic metastasis was 24(17.4%). Non-hepatic metastases were detected in 22(15.9%) patients. Eight (5.8%) patients were found to have both hepatic and non-hepatic metastasis. The most common finding as non-hepatic metastases was intraabdominal implants (10 patients, 7.2%).
Conclusion: In patients with suspected hepatic metastasis, DW-MRI imaging is a very useful and easily used method.
Keywords: diagnosis of suspicion metastasis, diffusion-weighted magnetic resonance imaging (DW-MRI)
DW-MRI, diffusion-weighted magnetic resonance imaging; CT, computed tomography; FDG-PET, fludeoxyglucose positron emission tomography; WB-DWI, whole-body diffusion-weighted imaging; DWI, diffusion weighted imaging; ADC, apparent diffusional coefficient; TRG, tumour regression grading; SS-EPI, single shot echo-planar sequence; SPAIR, spectral attenuated inversion recovery
The liver is one of the most common organs in which metastases from malign tumors occur. Liver metastases from malignancies are about 18–40 times more common than primary liver tumors.1,2 For instance, in colorectal carcinoma, which is the third most common tumor worldwide, liver metastasis develops in up to 50% of patients.3 As in colorectal carcinomas, liver is the most common organ of metastasis in pancreatic cancer.4 In recent years, the treatment plans have been successfully developed for patients with massive liver metastases.5 The presence and pattern of liver metastases are very important considerations when making treatment plans, especially for surgical decisions.6
Surgical resection is the most important treatment method in liver metastasis patients. Generally the first treatment option in both primary and secondary malignant liver lesions is surgery.1,3 At the same time, treatment of liver metastasis in cancer patients has seriously affected the life span positively.7 Surgical strategies have a wide spectrum, including segmentectomies, multisegmentectomies, metastasectomies, and atypical resections.8 Other treatment methods such as intraoperative ablation or preoperative endovascular embolizations with or without chemotherapy can be considered as an option.9,10 Detection of liver metastases is not only vital for sufficient surgical treatment but also for the decision of the best treatment such as resection, neo-adjuvant chemotherapy, or palliative treatment.11 Preoperative imaging to determine an accurate mapping of the tumors is important when considering the morbidity of liver surgery, especially in incompletely treated patients.
Numerous non-invasive preoperative methods for detection of liver metastases have been proposed and described. These include computed tomography (CT), magnetic resonance imaging (MRI), Fludeoxyglucose positron emission tomography (FDG-PET), and contrast enhanced ultrasonography.11 Recently, with the development of new MRI scanner and coil technology, whole-body diffusion-weighted imaging (WB-DWI) has been recognized as a new imaging modality for the assessment of metastases of various malignancies without radiation exposure and motion artifact.12
Diffusion weighted imaging (DWI) is a Magnetic Resonance Imaging (MRI) sequence acquisition that is sensitive to free water diffusion. Regions of reduced extracellular space due to high cell density or other micro-environmental factors result in restricted diffusion of free water relative to the surrounding tissue. Similarly, increases in extravascular-extracellular space due to cell death may result in increased free water diffusion. Consequently, apparent diffusional coefficient (ADC), derived from diffusion weighted MRI, has received considerable attention as a potential biomarker of early response to cytotoxic therapies.13
Diffusion-weighted MRI (DWI) is sensitive to the molecular diffusion of water in biologic tissues, and recent technical advancements permit obtaining high quality DWI images of the liver. Colorectal liver metastases show high signal restricted diffusion on DWI compared with normal liver parenchyma. Several studies have indicated the potential usefulness of diffusion-weighted MR imaging (DW-MRI), focusing on the added value of ADC semi-quantitative analysis, to distinguish patients with major metastatic response to chemotherapy (responders) from patients without any response (non-responders). However, in those studies, a reference pathological examination using the tumour regression grading (TRG) system was not performed.4
Therefore, the purpose of our study was to prospectively compare the diagnostic performance of DW imaging, gadoxetic acid–enhanced MR imaging, both techniques combined, and CT for detection of colorectal hepatic metastases and to evaluate the incremental value of MR imaging for patients with potentially curable colorectal hepatic metastases detected with CT. With recent technological improvements, radiologists can obtain high quality DWI images of the liver metastases and show high signal due to restricted diffusion on DWI compared with surrounding tissue.
DW-MRI technique is important method for determination of liver metastases and subsequent treatment decision. DW-MRI is one of the methods that provide important information.14–17 The purpose of our study was to prospectively compare the diagnostic performance of DW-MRI for detection of hepatic metastases and to evaluate the incremental value of DW-MRI for patients. Also, in some studies, contrast agents have been used to increase the efficiency of DW-MRI technique. In our study, we aimed to elucidate liver metastases easily, and thus DW-MRI was performed without additional contrast agent.
Patient characteristics
A total of 138 patients with cancer were evaluated. Median age was 60 years (range: 17–85). Of these, 55.8%(77) patients were female and 20.3%(28) patients had breast cancer (Table 1). All patients had remaining suspicion of liver metastasis. For this reason, all patients (100%) underwent DW-MRI. Before DW-MRI, all the patients (100%) had upper abdomen computerized tomography scan. PET-CT was performed in 98(71.0%) patients (Table 1).
Characteristics |
n(%) |
All |
138(100.0) |
Gender |
|
Female |
77(55.8) |
Age |
|
≥65 |
49(35.5) |
Upper abdomen CT scan |
138(100.0) |
DW-MRI |
138(100.0) |
PET-CT |
98(71.0) |
Upper abdomen MRI |
29(21.0) |
Upper abdominal USG |
24(17.3) |
Breast cancer |
28(20.3) |
Colorectal cancer |
22(15.9) |
Lung cancer |
18(13.0) |
Ovarian cancer |
15(10.9) |
Pancreatic cancer |
7(5.1) |
All other cancers |
48(34.7) |
Table 1 General characteristics of the patients
CT, computerized tomography; DW-MRI, diffusion-weighted magnetic resonance imaging; PET-CT, positron emission tomography-computerized tomography; MRI, magnetic resonance imaging; USG, ultrasonography
Evaluation of DW-MRI
All MRI examinations were performed with the same protocol, using a 1.5-T clinical MRI unit (Magnetom Avanto®, VB15 software version, Siemens Healthineers). Axial DW-MRI was acquired through the entire liver using a single-shot spin-echoecho-planar sequence (SE-EPI) with multiple b-values (50,400,800s/mm2), parallel imaging technique and with diffusion-weighted gradients applied in all the three orthogonal directions. DWI parameters were as follows: b-value images for 3 orthogonal gradient directions, 4 signal averages per image, free-breathing single shot echo-planar sequence (SS-EPI), spectral attenuated inversion recovery (SPAIR) fat suppression, 4.5mm axial slice thickness, 40 slices with no inter-slice gap, target FOV of 380mm, bandwidth 1400–1800Hz per voxel size of 1.4 x1.4x4.5mm. MR images were analyzed by one radiologist with 5 years’ experience in abdominal MRI.
Evaluation of CT
All patients underwent either quadruple-phase MDCT consisting of early arterial, portal venous, and delayed venous phases using section thicknesses of 1mm. A total of 1.5ml of non-ionic contrast material (Iobitridol 350mgI/ml, Xenetix®, Guerbet, Paris, France) per kilogram of body weight (520mgI/kg.bw) was administered for 30s via a power injector (Multi level CT; Medrad, Pittsburg, Pa) at a rate of 2–5ml/s using an 18- to 20-gauge intravenous catheter in the antecubital vein, followed by a 20- to 30-ml sterile saline flush. For image acquisition, the automatic bolus tracking technique was used. The trigger threshold was 100HU at the abdominal aorta. The average imaging time delay was 24s for the early arterial phase, 70s for the venous phase, and 180s for the delayed venous phase.
CT images were analyzed by one radiologist with 5 years’ experience in abdominal MRI.
Statistical analysis
Statistical data analysis was performed using the Special Package for the Social Sciences (SPSS) version 16.0 for Windows (SPSS Inc., Chicago, Illinois, USA). Frequencies analysis was used. P values of <0.05 were accepted as statistically significant.
Malignancy signs were detected in 46(33%) of the patients with DW-MRI whose metastasis was not detected by all other tests. The number of patients with newly detected hepatic metastasis was 24(17.4%). Non-hepatic metastases were detected in 22(15.9%) patients (Table 2). We compared the DWI and CT images; DWI images clearly showed additional benefits over CT alone (Figure 1-3).
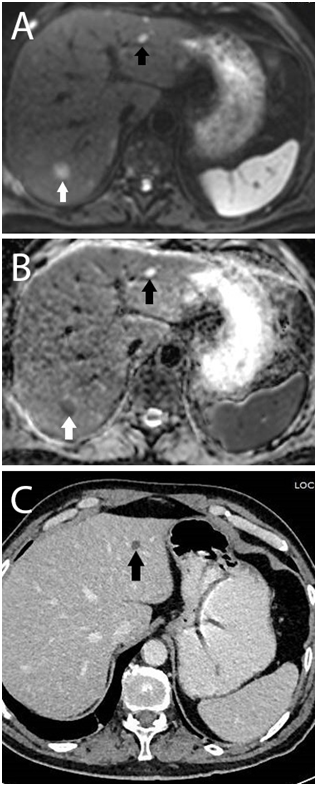
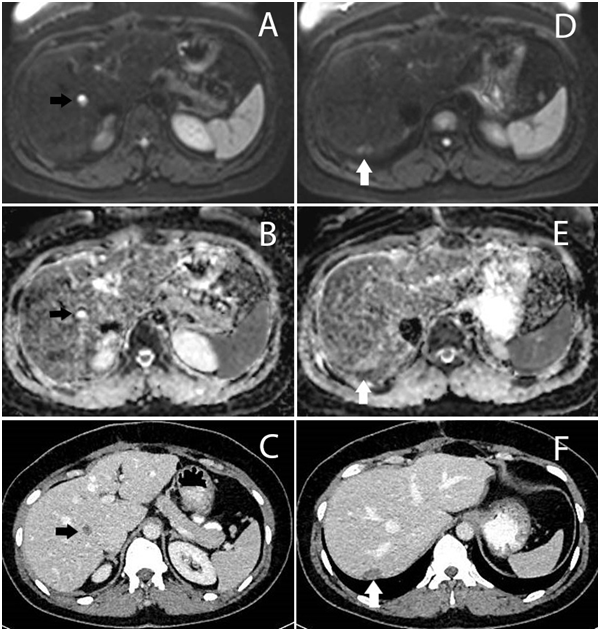
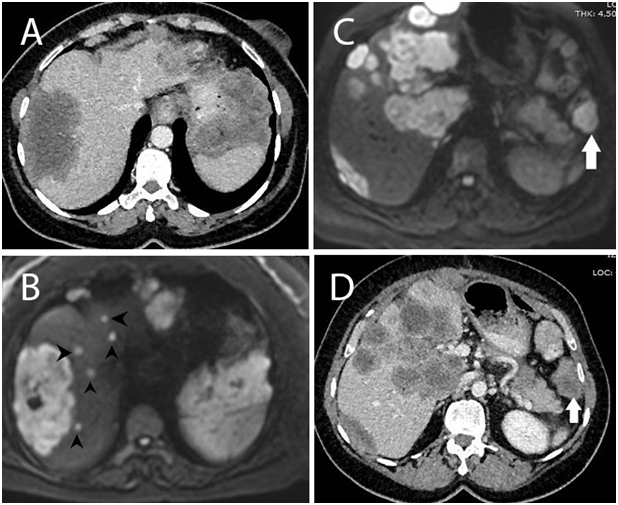
Characteristics |
n(%) |
All patients |
138(100.0) |
Liver metastasis suspected |
138(100.0) |
Malignancy signs detected with DW-MRI but not detected by all other tests |
46(33.3) |
Non-hepatic metastases detected |
22(15.9) |
Hepatic and non-hepatic metastases detected |
8(5.8) |
Intraabdominal implants detected |
10(7.2) |
Table 2 Results
Pts, patients
Liver metastasis is very crucial point for decision to treatment schedule such as surgery, or neo-adjuvant chemotherapy or palliative treatment. For this purpose, we performed diffusion-weighted MR imaging (DW-MRI). Also we were applied without giving any medicine to the patient.
In our trial using DW-MRI imaging, malignancy signs were detected in 46(33%) of the patients whose malignancies were not detected by all other tests. The number of patients with newly detected hepatic metastasis was 24(17.4%) and non-hepatic metastasis was 22(15.9%) patients. The most common finding in non-hepatic metastases was intra-abdominal implants (10 patients, 7.2%).
Ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), DW-MRI, positron emission tomography (PET) are choices for the detection of hepatic metastases. These methods are useful for hidden metastases.12,15,18 The determination of liver metastases and subesquent treatment decisions are important. DW-MRI technique, which is one of the methods used for this purpose, provides important information. In some studies, contrast agents have been used to increase the efficiency of DW-MRI technique.14,16,17
USG, multi-phasic contrast enhanced CT, PET-CT and MRI can be used to detect unspecified liver metastases. Each has its own advantages and disadvantages. Here, DW-MRI technique is easy to apply and can be applied to the patient without contrast agent. In addition, its efficacy in revealing hidden metastases has been shown.19 Yamada and his friends first showed that employing DWI could be beneficial in discriminating hepatic lesions in 1999.20 Since then, efforts have been undertaken to extend DWI to oncological applications involving other organs. As a result, DWI has been increasingly incorporated in routine clinical imaging as an adjunct MRI technique and has shown great promise for tumor detection, staging, monitoring and predicting treatment response in various organs.21,22
DW-MRI technique with contrast agent has been used and found to be effective in cases with suspected liver metastasis.3,23 In some studies, the usefulness of DW-MRI technique without contrast agent was shown.24 In our study, we used DW-MRI technique and found that it elucidated liver metastases without additional contrast agent.
Our study has some limitations. The important limitation was small number of cases. Another limitation was that MRI, USG and PET were not performed in all patients.
However, considering the importance of undiagnosed liver metastases, it is seen that DW-MRI method is a very important technique. The purpose of our study was to prospectively detect suspected liver metastases easily with DW-MRI without use of contrast agent. We suggest that the DW-MRI method is an easily applicable and available method.
We would like to acknowledge the www.makaletercume.com for their outstanding scientific proofreading and editing services that was provided for this manuscript.
The author declares that there is no conflicts of interest.
No
None.

©2019 Yirgin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.