International Journal of
eISSN: 2574-8084


Research Article Volume 5 Issue 4
1Department of Radiorherapy, Instituto Nacional Oncologia y Radiobiologia, Havana, Cuba
2Hospital Universitario "Juan M.Marquez", Havana, Cuba
3Department of Clinical Research, Instituto Nacional Oncologia y Radiobiologia, Cuba
4Department of Oncopediatric, Instituto Oncologia y Radiobiologia, Cuba
Correspondence: Jose Alert, Department of Radiorherapy, Instituto Nacional Oncologia y Radiobiologia, Havana, Cuba
Received: December 08, 2017 | Published: August 28, 2018
Citation: Alert J, Chon I, Valdes J, et al. Long term survival in diffuse infiltrative brainstem gliomas in children and adolescents treated with radiotherapy and Nimotuzumab. Int J Radiol Radiat Ther. 2018;5(4):267-270. DOI: 10.15406/ijrrt.2018.05.00176
Background: Brainstem gliomas have a short survival time; chemotherapy has not improved outcome. We report the result obtained with the combination of Radiotherapy and the monoclonal antibody Nimotuzumab in a series of these tumors.
Material and methods: 40 children and adolescents treated with irradiation and Nimotuzumab were included between Jan 2009 and December 2015, all with the diagnosis of diffuse infiltrative pontine gliomas (DIPG), irradiated at the Instituto Nacional de Oncologia y Radiobiologia, in Havana, Cuba. Nimotuzumab was applied during the period receiving Radiotherapy and them monthly for one year or more.
Results: Median age at diagnosis was 7,9 years (range 3-18 years old); median survival was 18,4 months and Kaplan Meier survival was 42,5% at 2 years and 34,5% at 5 years, established till 9 years. Addition of Nimotuzumab was safe and well tolerated.
Conclusion: Combination of Nimotuzumab and Radiotherapy is safe and could increase survival.
Keywords: radiotherapy, nimotuzumab, brainstem gliomas in children and adolescents
The brainstem is defined as the pons, midbrain and medulla oblongata, and 70% to 80% of the tumors in this localization are diffuse intrinsic pontine gliomas (DIPG). They typically present short duration of symptoms (multiple bilateral cranial nerve deficits, specially VI and VII, and also ataxia) and characteristic appearance in imaging finding: histological confirmation has not been required;1–4 there has been discussion about the histological diagnosis by biopsy.5,6 Radiation therapy (RT) has remained the standard treatment with a low degree of efficacy and short term responses, generally with a 1 or 2 year median overall survival.1,3,7–11 It have been attempted different strategies in order to improve the prognosis, which included the association of chemotherapy and also hypofractionation12–19 but they not improved survival, and recent biologic drugs are combined with RT.2,14,20–23
We tested the hypothesis that the combination of RT with Nimotuzumab, a humanized monoclonal antibody (MAb) developed at the Center of Molecular Immunology, in Havana, Cuba, can improve the survival in DIPG. The antibody was obtained by humanization of the murine antibody EGF/R324 As Nimotuzumab has a 10 fold affinity to EGFR as compared with Cetuximab; its capacity to bind EGFR is heavily dictated by cell receptor density.25 Nimotuzumab preclinical and clinical characterizations have been summarized before,16–18 and a distinguishing feature compared with other MAb is the lack of severe skin toxicity and the possibility to be used beyond progression. 20,23,25.
This is a prospective, non-randomized clinical study conducted to assess the efficacy of the combined treatment of RT and Nimotuzumab in 40 diagnosed DIPG patients between 3 and 18 years old, included from Jan/2009 to Dec/2015 and followed continued till Dec/2017, or to the death of the cases, with a median follow-up of 46 months. Ethical Committee approved this study and a signed informed consent was obtained from patients or legal guardians before starting treatment. Diagnosis criteria was radiological finding (CT or MRI) of DIPG infiltrative lesion; involving 2/3 of pons and at least 2 of the 3 classical neurological signs and symptoms (cranial nerve palsy, corticospinal tract deficit and ataxia) lasting less than 6 months, and a Karnofsky Lansky performance status not less than 40. It was a histopathological confirmation in 3 patients. During this period no one DIPG was excluded from treatment.
Eligibility criteria were: Patients must be older than 3; follow-up no less than 6 months, Karnofsky Lansky performance status not less than 40. Patients with focal lesions of the brainstem were not eligible for this study, because focal tumors have a better prognosis.14,26–29
Linear Accelerator was used for the radiation treatment, Gross Tumor Volume (GTV) was defined as the visible tumor, either by MRI or CT, Clinical Target Volume (CTV) accounted for subclinical microscopic disease and unappreciated tumor extension generally 1,5 cm beyond GTV planning, Planned Target Volume (PTV) was 0,3-0,5 cm beyond CTV, and could vary according to de Organ at Risk . Rt dose range from 54 to 59, 8Gy with a dose per fraction of 1,8Gy. Four patients were planned with IMRT and the rest with 3DCT.
Application of the Nimotuzumab begins with the first irradiation, and it was administered at 150mg/m2 IV, weekly during the term of the RT, then every 2 weeks for 8 doses, then monthly for one year. The last patients included with Nimotuzumab the time of application was prolonged for 2 years.
Thirty-four patients (85%) received the complete Nimotuzumab schema, and in 6 there were minor interruptions of the dosage applied because these patients did not concur to the treatment options in a timely manner. Two patients were reirradiated because of local relapse. Simulation, contouring and planned procedures was done with the same parameters of first irradiation, with doses of 54 and 52Gy.
After irradiation the patient were follow every month till one year, then every 3 months, or till the moment of death.
The objective respond was evaluated according to the RECIST criteria. The same radiological technique for the initial diagnosis was used. Complete response (CR) plus Partial Response (PR) plus Stable Disease (SD) was considered acceptable with a response rate of 80% or more. Characters of the series are in Table 1.
Median age at diagnosis |
7,9 ( range 3-18 ) |
Male/female ratio |
1,2:1 |
Type of tumor DIPG |
|
Localization |
|
Midbrain and pons |
26 |
Pons |
8 |
Cerebello-pontine |
6 |
Table 1 Characters of the serie
Statistical methods
Descriptive statistics were reported as absolute frequencies and percentages for qualitative data, whereas medians and ranges were used for continuous variables. Relapse and death (whatever the cause) were considered events. Local control was defined as the absence of relapse in the irradiated primary tumor site RT field. Local control, event-free survival, and overall survival rates were estimated by the Kaplan–Meier method, and differences between groups were assessed by the log-rank test. Survival estimates refer to times of 5 years after diagnosis, and the related 95 % confidence intervals (95 % CI) were calculated all statistical tests were two-sided and a p-value of < 0.05 was considered significant. Analyses were performed using SPSS V21.
Median age of the cases at diagnosis was 7,9 years (range between 3 and 18 years old). In all cases tumor extended to the pons and in one case to 4th ventricle, cerebellum and hypothalamus. The diagnosis was made by clinical and imaging findings, but in 3 cases it was possible a pathological examine, one with astrocytoma Grade II and 2 others astrocytoma Grade III, all of them alive. Median overall survival was 18,4 months, 95% CI (14.2-23.7) and Kaplan Meier accumulated was estimated in 42,5 % at 2 years, 34,5 % at 5 years and established till 9 years (Figure 1).
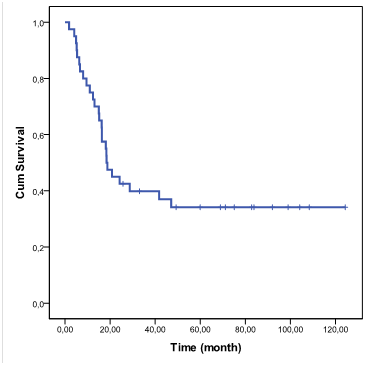
Figure 1 Overall survival function. DIPG in children and adolescents treated with radiotherapy and Nimotuzumab.
At this moment (Dec/2017) there are alive 14 patients:2 patients with 9 years, 2 for 8 years, 1 for 7years, 3 with 6 years, 2 with 5 years, 2 for 4 years, and 2 for 2 years. Two patients were reradiated because of relapse, they are one alive at 6 years and the other death in the second year:
Univariate analysis showed no significant differences according to sex, age and dose of irradiation, but we notice a tendency to best survival in 10-14 years age group. The combined treatment was well tolerated and no grade III or higher grade was observed. Adverse events such as vomiting, headache, fever, etc were reported in 40% of patients. Alopecia in all the irradiation fields was present in 36 patients (92,2%). At the end of irradiation there was a clinical response (CR or PR) in 39 patients (97,5 %). Only one patient had no response to radiation treatment.
DIPG comprise a group of tumors with a generally bad prognosis despite initial response to irradiation, which is the ideal and accepted treatment for those tumors, with a median survival of one year.1–4,7–11,17 Neither Chemotherapy or hypofractionation12–18 nor irradiation dose escalation have improve survival, but increase toxicity.
We tested the hypothesis that Nimotuzumab concomitant with RT will increase survival in DIPG. Nimotuzumab is a humanized monoclonal antibody, EGF/R3,21,24,25,30 with a 10 fold affinity to EGFR. It has been reported that there are overexpression and of ERRB1/EGFR,31,32 in DIPG suggesting that this tyrosine-kinasa receptor might be a target. In other reports EGFR is overexpressed in DIPG, suggesting that EGFR dysregulation could be a therapeutic target.24 Blood-brain barrier is crossed.33,34 In high grade gliomas, after Rt and Nimotuzumab it has been pound monoclonal antibody uptake in residual lesions.33
We compared the present series with a first group of DIPG children’s treated only with RT in the period 1992-2008 in the same institution (National Institute of Oncology and Radiobiology, in Havana), since there were no major clinical differences between the groups of patients (data not shown): in this first group of patients treated between 1992-2008 median survival time was 10 months, and the survival rate at 2 years was 15%.2 A previous report with the use of Nimotuzumab and Rt in the same institution, that included only 28 patients from 2009 to 2012 showed a survival rate at 3 years of 39,7%.2 The present report include the anterior patients and new patients with a total of 40 patients and a period of time extended from 2009 to 2017, with a 2 year survival of 45,5% and 34,5 at 5 years , and stablished after that period till 9 years.
In a recent report from Massimino et al.,3 of DIPG in children treated with Nimotuzumab, vinorelbine and radiation and reirradiation, the 2 years overall survival was 27 +-9%, supporting the use of Nimotuzumab in combination with RT.
We have 2 patients reirradiated, both with the application of Nimotuzumab: one received radiation treatment at the second year of evolution, and is a alive and apparently controlled after 9 years; the second at the relapse in the first year, and is death at the second year of the reirradiation. Nimotuzumab is safe and effective with a lack of skin toxicity and with the possibility to be used beyond progression.2,3,20,25,34,35 In this series there is a tendency to better survival in 10-14 years old group: on other reports a better outcome is observed in younger children.3,34 Increased dose over 57Gy didn’t demonstrate better results.
Survival from DIPG irradiatedin children and adolescents is poor, no matter the use of Chemotherapy associated. But the use of RT and Nimotuzumab is a promising therapeutic option that can increase overall survival and support new trials with therapeutic combination. Reirradiation could be valuated in cases of relapse.
No potential conflict of interest exists.

©2018 Alert, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.