International Journal of
eISSN: 2574-8084


Case Report Volume 5 Issue 3
1Department of Radiation Oncology, Mater Sydney Hospital, Australia
2Faculty of Medicine, University of New South Wales, Australia
3Department of Dermatology, Concord Repatriation General Hospital, Sydney, Australia
4Department of Radiation Oncology, St Vincents Hospital, Australia
5Faculty of Engineering, University of Technology, Australia
Correspondence: Gerald B Fogarty, Department of Radiation Oncology, St Vincents Hospital, Victoria St, Darlinghurst 2010 NSW, Australia, Tel +612 83025400
Received: May 07, 2018 | Published: May 14, 2018
Citation: Martin TD, Moutrie Z, Tighe D, et al. Volumetric modulated arc therapy (VMAT) for skin field cancerisation of the nose - A technique and case report. Int J Radiol Radiat Ther. 2018;5(3):142-148. DOI: 10.15406/ijrrt.2018.05.00152
Locally advanced skin cancer of the nose is problematic, especially when all nasal skin needs treatment as in the case of skin field cancerisation (SFC). Surgery is considered the gold standard but involves tissue loss which may impact function and cosmesis. In patients who are not good surgical candidates, definitive radiotherapy (RT)can be used.
Traditional RT modalities for the nose, such as brachytherapy, superficial x-ray treatment or electrons, have quality assurance issues and can be difficult to conform to the target volume. Volumetric modulated arc therapy (VMAT) is an advanced form of intensity modulated radiotherapy (IMRT). IMRT confines the high dose of radiation to the tumour and is used to avoid irradiating a dose-sensitive structure in the concavity of a volume that requires a therapeutic dose for tumour control. Within the nose, the nasal septum is a dose-limiting structure in the concavity of the skin covering the nose.
We present here a case report using a VMAT technique to treat SFC of the nose. To our knowledge, this is the first description in the literature of VMAT for SFC of the nose. We also believe it to be the first time that VMAT simultaneous integrated boost (SIB) to biopsy proven macroscopic disease has been used when treating SFC.
Keywords:basal cell carcinoma, case report, radiotherapy, simultaneous integrated boost, skin cancer, volumetric modulated arc therapy (VMAT)
BCC, basal cell carcinoma; cGy, centi-gray; CTV, clinical target volume; CT, computed tomography; EBRT, external beam radiotherapy; Gy, gray; GTVs, gross tumour volumes; IMRT, intensity modulated radiotherapy; Mm, millimetre; NMSC, non-melanoma skin cancer; OAR, organ at risk; PTVs, planning target volumes; RO, radiation oncologist; RT, radiotherapy; RCT, randomised controlled trial; SIB, simultaneous integrated boost; SFC, skin field cancerisation; SXRT, superficial radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy technique; TPS, treatment planning system; VMAT, volumetric modulated arc therapy
Therapy for locally advanced skin cancer of the nose is problematic. Surgery involves tissue loss which may have an impact on function and cosmesis. Definitive radiotherapy (RT) can also be used without tissue loss. RT involves fractionation with multiple visits to the treatment centre. RT can also cause side effects in normal and surrounding tissues within the treatment volumes. A randomised controlled trial (RCT) in basal cell carcinoma (BCC) treated with either surgery or RT found that there was no significant difference in the cosmetic outcomes between surgery and RT when the tumour was located on the nose.1
Volumetric modulated arc therapy (VMAT) is an advanced form of intensity modulated radiotherapy (IMRT). IMRT has been used in various parts of the body to avoid irradiating a dose-sensitive structure in the concavity of a volume requiring a therapeutic radiation dose for tumour control.2 In the event of skin field cancerisation (SFC) of the nose, all nasal skin needs treatment. Within the concavity of the skin covering the nose is the nasal septum, which is a dose limiting structure. Traditional techniques do not allow sparing of the septum, causing morbidity which may lead to inadequate treatment.
We hypothesized that VMAT could be a solution to SFC of the nose for whom therapies like surgery and tradition radiotherapy were considered too morbid or technically challenging. We present here a case report using a VMAT technique to treat SFC of the nose. To our knowledge, this is the first description in the literature of VMAT for SFC of the nose. We also believe it to be the first time that VMAT simultaneous integrated boost (SIB) to biopsy proven macroscopic disease has been used to treat SFC.
A 75-year old healthy Caucasian male with biopsy proven multifocal basal cell carcinomas (BCCs) of both lateral nasal alae and bridge of nose was referred for definitive RT. There was no history of immunosuppression or prior therapy to these lesions, nor any symptoms or clinical signs of perineural invasion, nor any evidence of more distant spread, including lymphadenopathy. He was on not regular medications and had no allergies. There was also no suggestion of a genetic predisposition to multiple BCCs.
The result of a multidisciplinary discussion was to treat the whole nose for SFC using VMAT with SIB to the palpable and biopsy proven gross disease. Figure 1 shows the planning marks for the separate palpable tumors with a five millimetre (mm) radial expansion for RT planning purposes.
Planning
The patient consented to treatment and was marked up for planning and treatment by the radiation oncologist (RO). Planning photos (Figure 1A-C) and a template (Figure 1D) were taken. The patient was placed on a computed tomography simulator (Siemens Somatom® Definition AS) and immobilised in a thermoplastic mask. The mask was placed so that it was flush against the nose with no air gaps. The planning marks were transferred onto the mask. Tissue equivalent material called ‘build up” or “bolus’ is necessary to ensure full dose to the skin when using megavoltage radiotherapy. Custom build up was made to cover the whole field using a special thermoplastic VMAT bolus material that, after construction and molding, dried to become a rigid mold of the target area. The planning marks were transferred from the mask to the surface of the bolus to assist computer-aided contouring (Figure 2A-B).

Figure 1 Biopsy proven multifocal basal cell carcinomas on both lateral nasal alae and bridge of nose seen at radiotherapy planning. Note that the nostrils were packed.
Figure 1A Anterior photo showing complete black line around the visible and palpable bridge of nose disease. Dotted line is 5mm expansion.
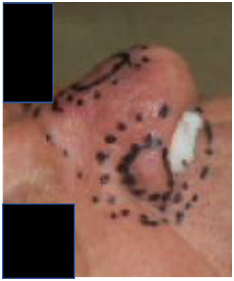
Figure 1C Lateral photo from the right side showing the lateral lesion on the nasal ala. The dotted fields are also appropriate expansions for planning target volumes (PTV) for VMAT.

Figure 1D A template was made by tracing the planning marks onto a flat sheet of perspex which was stored in the electronic medical record (EMR). The template aided daily treatment set up and documented the area that was treated.

Figure 2A The marks made on the nose were transferred onto a personalised thermoplastic immobilisation mask.

Figure 2B Prior to mask construction, ‘wet gauze’ bolus was placed in both nostrils for dose distribution reproducibility in the nostril air cavities. The thermoplastic bolus was then constructed conforming to the defined target. Two sheets of thermoplastic bolus were used to achieve the desired 7mm thickness. This was necessary to build up the dose in the bolus material to ensure full dose to the skin. Once dried, the thermoplastic mould was rigid. The target volume outlines were then transferred onto the bolus with a pen and then wire to aid with contour delineation on the resulting planning CT.
Figure 2 Planning.
Wires were placed on the planning marks to ensure they were captured onto the resulting planning computed tomography (CT). A planning non-contrast CT was taken with two mm slices from the vertex of the skull to the shoulders. The gross tumour volumes (GTVs)3 were contoured in the treatment planning system (TPS; Varian Eclipse™ v11.0.3) based on the wires placed at simulation. Planning target volumes (PTVs)3 of each GTV were added also using the wires that were marking the five mm expansion. The nasal skin was also contoured as the clinical target volume (CTV).3 Figure 3 shows the computer contouring by the prescribing RO.
The prescription was a typical SIB prescription consisting of 60Gy in 25 fractions for the PTVs of each GTV, and 50Gy in 25 fractions for the PTV of the nasal CTV. A six megavoltage (MV) VMAT plan was created. Figure 4 shows the RT planning. A traditional three-dimensional conformal radiotherapy technique (3D-CRT) with an opposed 6 MV photon plan that irradiated the whole PTV of the nasal skin was also created and compared at different levels of dose in axial and sagittal planes.
The nasal septum was also contoured as an organ at risk (OAR).3 The dose constraint put on the septum was “mean dose less than 30Gray (Gy)”. This was thought to be the cut-off point for the development of serious acute mucositis that may impact on treatment continuity. The differences in dose to the septum in the two plans were calculated and compared on a dose - volume histogram. The mean dose received by the septum from the VMAT plan was 61% less than the dose of the 3D-CRT plan, while the maximum septum dose from the VMAT plan was 20% less than the 3D-CRT plan.
To test the dosimetry accuracy of the delivery of the VMAT plan, a phantom was used. The Alderson Radiotherapy Anthropomorphic phantom was simulated with the same thermoplastic mask material; two layers of thermoplastic bolus were made and placed on the surface of the nose and cheeks, a CTV and three GTVs (intended SIBs) were delineated on the bolus, and radiopaque markers were placed at the target locations (Figure 5B) to enable contouring in the TPS. The phantom in mask was scanned and sent to Eclipse™ v11.0.3 for contouring and planning. GTV and CTV were contoured, and the same prescription dose as that intended for the patient was applied; the pertinent OARs were delineated and an acceptable VMAT plan was created. Pieces of radiochromic film were placed at key locations on the phantom to verify the dose on the surface (Figure 5A). Due to the high dose gradients in this anatomical region and the integrated boost prescription, each dosimeter exhibited regions of high and low dose as shown in Figure 5C and indicated in Table 1.
Measured |
Eclipse™ v11.0.3 |
|||
Low dose region (cGy) |
Hi dose region (cGy) |
Low dose region (cGy) |
||
Right ala |
180±15 |
120±15 |
180±15 |
120 ±15 |
Left ala |
165±10 |
115±15 |
170 ±15 |
125 ±15 |
Apex/inf dorsum |
172±5 |
120±20 |
168 ±10 |
123 ±20 |
Bridge |
170±8 |
40±15 |
170 ±15 |
42 ±15 |
Table 1 Variation in dosimeter readings measured on the phantom versus the Eclipse™ treatment planning system (TPS)
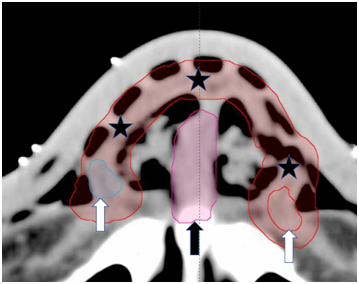
Figure 3 Contouring on axial slices of the planning CT by the prescribing radiation oncologist (RO). Note the wires on the surface of the bolus that faithfully reflect the marks made on the skin with a pen at simulation. The white arrows show GTVs. The red rind marked by black stars is the nasal CTV, and the pink central structure marked by a black arrow is the septal avoidance structure.

Figure 4.3B 3D-CRT plan. 30Gy dose wash which is a sufficient dose that may cause normal tissue side-effects.

Figure 4.4A VMAT plan. Midline sagittal dosimetry of septum avoidance structure (black arrows) at a dose wash of 30Gy. Notice the septum sparing here compared to Figure 4.4B.

Figure 4.4B 3D-CRT plan. Midline sagittal dosimetry of septum avoidance structure (black arrows) at a dose wash of 30Gy. Notice the dose to the septum compared to Figure 4.4A.

Figure 4.5 Dose volume histogram (DVH) of septum avoidance structure. Single black arrow denotes the dose received in the 3D-CRT plan. The double black arrow denotes the dose received in the VMAT plan. The mean dose received by the septum from the VMAT plan was 61% less than the dose of the 3D-CRT plan.
The variation between the measured and TPS calculated doses was most notable in the region of the phantom left ala. This variation may be attributed to some uncertainty in the physical set-up of the phantom and the high gradient region of the dose within the treatment plan (Figure 5C). The technique passed quality assurance. Radiation treatment was then delivered using the Varian Truebeam 2.0 linear accelerator. Gafchromic EBT3 radiographic film was used to perform in vivo dosimetry at key anatomical locations during treatment and demonstrated clinically acceptable agreement between the planned and measured dosimetry (Table 2). Figure 6A & 6B show the placement of the in-vivo recording devices.
Location |
Dose measured per fraction (Gy) |
Dose planned per fraction (Gy) |
Percentage difference (%) |
Anterior nose |
1.8 |
1.77 |
2 |
Left nose SIB |
2 |
2.08 |
-4 |
Left nose |
1.8 |
1.87 |
-4 |
Right nose SIB |
2 |
2.05 |
-2 |
Right nose |
1.8 |
1.78 |
1 |
Table 2 In vivo dosimetry during treatment
SIB, simultaneous integrated boost

Figure 5B Anthropomorphic phantom placed in simulation position with mask, bolus and delineating markers in place.
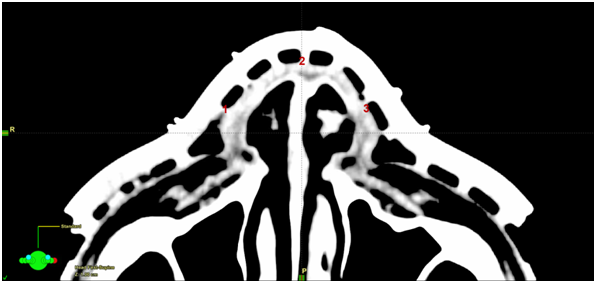
Figure 6A Planning scan showing placement of the in vivo dosimetry recording devices. 1=right nose; 2=anterior nose; 3=left nose.

Figure 6B Patient set-up showing placement of the in vivo dosimetry recording devices.
Figure 6 In vivo dosimetry was carried out during radiotherapy. See Table 1 for results.
Photos were taken in the last week of treatment showing the expected dry desquamation within the treatment field (Figure 7). All the prescribed treatment was delivered. Treatment was delivered without any break. At end of treatment, physical examination revealed a complete tumour response. There was persistent and resolving erythema and lymphoedema on the tip of nose, but the septum recovered completely. The radiation dose received to 50% of the volume of the septum using the VMAT technique was 28% of the dose had 3D-CRT been used, while the modal dose to the septum using the VMAT technique was 8% of the modal dose in the 3D-CRT plan.

Figure 7C Left lateral view.
Figure 7 Photos were taken in the last week of treatment showing the expected desquamation within the treatment field. The tumours exhibited a complete response on physical examination. There was persistent and resolving erythema and lymphoedema in the tip of nose, but the septum presented only erythema.
To our knowledge, this is the first description in the literature of VMAT for SFC of the nose. We also believe it to be the first time that VMAT SIB to biopsy proven macroscopic disease has been used to treat SFC. Our hypothesis that VMAT could be a solution to SFC of the nose for whom therapies like surgery and tradition radiotherapy were considered too morbid or technically challenging is working at this early stage. More time will tell if there has been significant sterilization of disease.
Australia has the highest number of skin cancers in the world.4 Non-melanoma skin cancer (NMSC) is the most common type of cancer in Australia and its incidence is increasing.5 In this country, NMSC accounts for the second highest cancer expenditure after colorectal cancer.6 Surgical excision is considered the ‘gold standard’ treatment for NMSC, and there is evidence to substantiate this.7–12 Surgery is often preferable for patients as it requires few visits to a medical practice, is highly effective, and yields a specimen for histological examination. However, surgery is not suitable for all patients. Non-surgical options may be preferred in those unfit for surgery, when tissue preservation is the primary concern, or where there are multiple superficial lesions that span a large area of skin.9,11–13
Radiotherapy may be an appropriate and equally efficacious option in patients who are not good surgical candidates.14 However, there is limited evidence comparing the efficacy of surgical excision and RT for NMSC.12,15 In 1997, Avril and colleagues8 conducted a randomised controlled trial (RCT) to observe the difference in local recurrence and cosmetic outcomes between surgery and RT for BCC on the face. This is the only RCT comparing local recurrence for these two modalities.10 Surgery yielded a lower four-year local recurrence rate compared to RT (0.7% versus 7.5%, respectively), and the cosmetic outcome was rated as ‘good’ by a five-judge panel in 87% of the surgery-treated patients compared to 69% of the RT-treated patients. This positions surgery as the superior treatment for BCC on the face for both local recurrence and cosmetic outcomes. However, only lesions less than four centimetres were included in the trial, and RT may prove to be a more effective treatment modality for lesions larger than four centimetres, like SFC. Furthermore, the RT techniques employed during the Avril study, whilst modern at that time, were not standardised and today’s techniques have significantly improved. Another RCT by Petit1 and colleagues comparing the cosmetic results of patients treated for BCC with either surgery or RT favoured surgery. Interestingly, for the sub group with nasal tumours, there was no significant difference in the cosmetic outcome between surgery and RT.
Radiotherapy techniques now available for SFC are well summarised by Fogarty et al.16 One of the traditional RT modalities for nasal NMSC is brachytherapy, but this involves expert creation and daily placement of a skin mould with no option for on-treatment verification.17 Other traditional types of external beam radiotherapy (EBRT) all involve compromise as the nose is a series of curved surfaces, and the traditional EBRT treatment volume is better represented by rectangles.16 Superficial radiotherapy (SXRT) can be used to treat lesions on the nose, but treating the whole nose is not possible due to field size limitations. Megavolt 3D-CRT includes opposed photon beams. This technique is ideal for the tip of nose, but the more the nose is involved, the higher the dose to the nasal septum which can lead to unacceptable mucositis and the need for a treatment break or cessation, affecting efficacy. Electron techniques can be used for the nasal alae. Every effort needs to be made to ensure that the tissue that is incident to the beam is as flat as possible and this usually involves taping the nose down, adding some uncertainty, and compromise. This results in questionable reproducibility of position, particularly when bolus material is needed to ensure that the skin receives the therapeutic dose.
Modern radiotherapy treatment modalities include intensity modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT). VMAT is an advanced form of IMRT with the benefit of a quick treatment time2 and no need for nasal distortion and taping, thus removing the problem of questionable reproducibility during treatment. VMAT in this case has aided completion of treatment in the planned time due to less mucositis on the spared septum. VMAT also allows the application of a SIB to gross biopsy proven disease within an area of SFC. We suggest that this technique be considered in adult patients with multiple NMSC on the nose needing treatment for which tradition therapies may be too morbid.
The authors wish to thank Aileen Eiszele for writing and editorial assistance, and Kristy Frappell for secretarial support.
The authors have no conflicts of interest to declare.

©2018 Martin, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.