International Journal of
eISSN: 2574-8084


Case Report Volume 4 Issue 6
1Trinity School of Medicine, USA
2Department of Pathology, University of Maryland Baltimore Washington Medical Center, USA
3Advanced Radiology, USA
Correspondence: Robert T Kidnie, Trinity School of Medicine, 10009 Woodkey Ln Apt 2, Owings Mills, 21117, USA
Received: December 04, 2017 | Published: December 19, 2017
Citation: Kidnie RT, Sopha SC, Sweet SM. A 59year-old male with anaplastic thyroid carcinoma. Int J Radiol Radiat Ther. 2017;4(6):466-468. DOI: 10.15406/ijrrt.2017.04.00118
Anaplastic thyroid carcinoma is a very rare and extremely aggressive carcinoma of the thyroid. Prognosis is dismal and by definition, is classified as stage IV upon diagnosis. Imaging plays a central role in defining the extent of disease, planning therapy, and monitoring the response to treatment. This case report illustrates the aggressive nature of anaplastic thyroid carcinoma and the value of imaging in guiding tumor biopsy, identification of metastases, and planning therapy.
Keywords: anaplastic thyroid carcinoma, computed-tomography, undifferentiated carcinoma, fine needle aspiration
ATC, anaplastic thyroid carcinoma; CT, computed tomography; ED, emergency department; HU, hounsfield unit; IR, interventional radiology; MRI, magnetic resonance imaging
A 59year-old man presented with dyspnea, dysphagia, and dysphonia developing over the course of one week. He was initially treated for pneumonia at an outpatient clinic severaldays prior, and his symptoms worsened considerably overnight prior to presentation at our institution. In the emergency department (ED), he showed thyromegaly. Chest X-ray was normal. His respiratory condition declined rapidly, prompting intubation. Computed tomography (CT) showed a very large retrotracheal and retrothyroid mass that extended into the mediastinum. Biopsy demonstrated anaplastic thyroid carcinoma (ATC).
ATC is an extremely aggressive and very rare tumor of thyroid follicular cells. Although it accounts for only 1.7% of thyroid carcinomas in the United States, it is responsible for up to 39% of thyroid carcinoma deaths. Prognosis is dismal: overall cause-specific mortality rate is 68.4% at 6months and 80.7% at 12months.1 As in our patient, common presenting symptoms of ATC include laryngeal, tracheal, and esophageal obstruction. Imaging is key for guiding biopsy, identifying metastases, and planning for surgery. This case underscores the aggressiveness of ATC and the role of imaging, particularly ultrasound and computed tomography, in the evaluation of the disease. We provide a summary of ATC to aid in future diagnosis.
A 59year old Caucasian male presented to the ED complaining of dyspnea, dysphagia, and dysphonia over the course of one week. Fivedays earlier, he had been evaluated at an outpatient clinic, diagnosed with pneumonia, and treated with azithromycin. His dyspnea and dysphagia acutely worsened in thehours prior to presentation at our institution, at which time he was visibly anxious, hyperventilating, and stridorous. Past medical history included Parkinson’s disease, hypertension, and hypothyroidism. Past surgical history included a minor herniorrhaphy. Both parents had died of cancer in middle age. The patient denied smoking.
While in the ED, the patient’s vital signs were 37.7°C, 85bpm, 156/101mmHg, 21resp, SpO2 95% on room air. Physical exam revealed an obviously anxious patient with an enlarged thyroid and mild resting tremor. Ativan was partially effective in relieving symptoms of anxiety. A chest x-ray demonstrated no acute pulmonary findings (Figure 1).
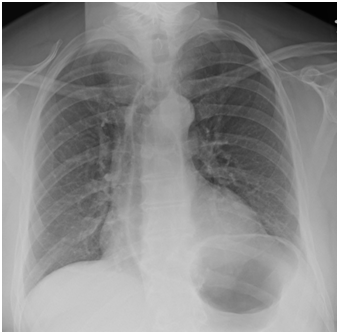
Figure 1 Chest x-ray ordered on presentation to the ED for shortness of breath. The image shows no acute pulmonary findings.
Over the course of less than 3hours in the ED, the patient became diaphoretic with diffuse wheezing and rhonchi requiring albuterol nebulizer. After intubation as recommended by the otolaryngologist, the patient was admitted to the intensive care unit. The initial CT (Figure 2A & Figure 2B) demonstrated a very large retrotracheal and retrothyroid mass measuring approximately 7 x 6 x 8cm, displacing the trachea anteriorly, the esophagus anterolaterally, and the thyroid anteriorly. It extended well into the mediastinum, nearly reaching the level of the carina, and considerably compressed the trachea at the level of the suprasternal notch and inferiorly. The thyroid gland was diffusely enlarged and contained multiple hypodense masses, the largest of which was 2.5cm, with an average density of 71 HU.
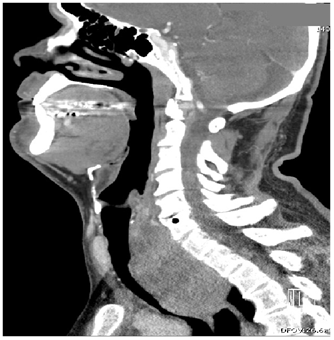

Figures 2A & 2B Sagittal and coronal CT scans of neck ordered prior to admission to ICU demonstrates a very large mass extending into mediastinum and compressing the trachea.
An ultrasound image of the right lobe of the thyroid obtained during fine needle aspiration (FNA) biopsy demonstrates complete loss of normal thyroid tissue architecture along the anterior portion of the right lobe, with punctate microcalcifications throughout. It also demonstrates heterogeneous areas suggestive of thyroid nodules within the thyroid gland (Figure 3).
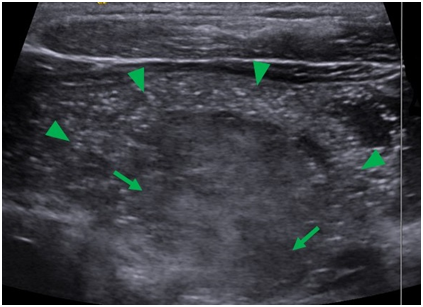
Figure 3 Sagittal ultrasound image of the thyroid during FNA. The image shows loss of normal thyroid architecture and contained within are diffuse microcalcifiactions (arrow heads) and heterogeneous areas suggestive of thyroid nodules (arrows).
Cytologic interpretation of the FNA specimen revealed anaplastic carcinoma of the thyroid with multinucleated giant cells. Focal CKAE1/3 expression supported the diagnosis of a carcinoma. Site-specific markers (TTF1, PAX8, Napsin, synaptophysin, chromogranin, Melan-A, HepPar, and CDX2) were all negative. Biopsy smears are presented as Figure 4A & Figure 4B. Given the extensive spread of the tumor in his neck, the patient was not deemed to be a surgical candidate. A CT of the chest, abdomen, and pelvis completed the patient’s staging, with no additional sites of disease.
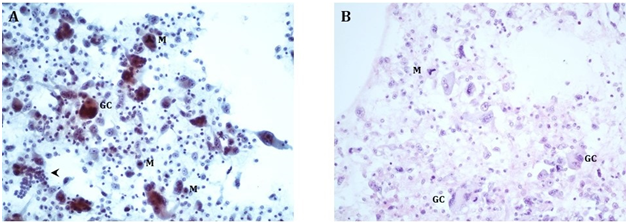
Figure 4A Direct smears, Papanicolaou stain, 200X, showing bizarre multinucleated osteoclast-like giant cells (GC) and neoplastic mononuclear cells with enlarged nuclei and prominent nucleoli. The tumor shows increased mitoses (M), including atypical ((tripolar) mitotic figures (upper center). For size comparison, a cluster of normal thyroid follicular cells is in the lower left corner (arrowhead).
4B Cell block, H&E stain, 200X, showing similar findings as the direct smears.
Twodays after presentation, the patient underwent emergent radiation therapy and began weekly carboplatin and paclitaxel therapy. After 12days, he had received two doses of chemotherapy with minimal sign of improvement. Onday 12, the patient developed thick yellow, blood-tinged secretions requiring suction. His clinical condition declined rapidly thatday, including two rapid-responses, and he died that afternoon.
Anaplastic thyroid carcinoma (ATC) is an extremely aggressive and very rare tumor of thyroid follicular cells. It accounts for just 1.3 to 9.8% of all thyroid cancers globally (1.7% in the USA) and has an overall age-adjusted incidence of 1 to 2 cases per million population peryear.2,3 Because of its rarity, epidemiologic studies typically encompass published series spanning several decades. One such study summarized the clinical features and outcomes of 1771 patients between 1949 and 2007. Men comprised 36% of the patients while women comprised 64%. The study found the median survival following diagnosis of ATC to be 5months, with a median 1year survival of 20%, making it one of the most aggressive solid tumors to affect humans.2 The median age of onset is 71.3years (SD 12.7years).1
In contrast to other types of thyroid cancer, ATCs consist of undifferentiated cells; however, approximately 20% of patients diagnosed with anaplastic thyroid carcinoma will present with a history of differentiated thyroid cancer; 20-30% of will have a co-existing differentiated cancer. Most of these differentiated thyroid cancers (papillary, follicular, Hürthle cell) tend to have a single mutation, whereas ATC commonly has multiple gene mutations.2
The most common initial clinical presentation of patients with ATC is the sudden growth of a longstanding goiter. Other common presenting symptoms include symptoms of laryngeal, tracheal, and esophageal obstruction.3 One retrospective study found that patients with ATC complained of dysphagia (40%), voice change (40%), and stridor (24%). The same study found patients presenting with a noticeable lymph node mass (54%) and neck pain (26%) as well as systemic symptoms. Less common symptoms include chest pain, bone pain, headache, confusion, and abdominal pain from metastases. Metastases were found in 50% of patients; the most common sites were the lungs (80%), bone (6-16%), and brain (5-13%).4
Diagnosis of ATC is typically made by cytologic examination of cells obtained by ultrasound-guided FNA biopsy. Where sample quality is inadequate and shows necrotic or inflamed tissue, core or surgical biopsy is indicated.5,6 The morphologic patterns of ATC include spindle, osteoclast-like giant cell (as in our case), or squamoid, and less frequently, a combination of these three patterns.7 Anaplastic (or dedifferentiated) carcinoma with osteoclast-like giant cells can arise in other organs, such as the pancreas, breast, stomach, bladder, or prostate. In nearly all such tumors, CD68 will mark osteoclast-like giant cells, while the neoplastic mononuclear cells will express at least one keratin marker (CK AE1/3, CAM5.2, EMA, or individual keratins), and, if sarcomatoid, vimentin. Lineage-specific markers are generally lost with dedifferentiation, and determining the organ of origin is dependent on identifying the co-existing differentiated carcinoma and imaging findings demonstrating the location(s) of the mass(es), rather than relying on immunohistochemistry. BRAF V600E mutation is found in up to nearly 25% of anaplastic thyroid carcinomas.8
Evaluation of patients diagnosed with ATC include detailed laboratory and imaging studies. Laboratory evaluation includes a detailed thyroid function test, blood chemistry evaluation and complete metabolic panel.6,4 Imaging studies are ordered to determine the extent of the disease, plan therapy and monitor response to treatment. The American Thyroid Association (ATA) recommends a high-resolution ultrasound of the neck for rapid evaluation of the tumor if not already performed. Cross-sectional imaging of the neck and chest with magnetic resonance imaging (MRI) and/or CT scan is also imperative to determine the presence of regional disease and exclude distant metastasis. For better evaluation of the extent of disease, CT scan with intravenous contrast will be helpful. Alternatively, MRI using gadolinium contrast can evaluate the neck and superior mediastinum. Positron Emission Tomography is useful in evaluating metastases.6 CT increases diagnostic accuracy and can indicate a suitable site for biopsy; it is indispensable in the planning of surgery.9
All ATCs are considered stage IV cancers. ATC limited to the thyroid are staged as IV-A, while ATCs with extrathyroidal extension are IV-B and distant metastases are IV-C. Treatment is determined by ATC stage and the tumor’s resectability. Depending on the extent of the tumor, options include surgical resection, locoregional radiation, systemic chemotherapy, and palliative care. Specific chemotherapy regimens are evolving with new research and are beyond the scope of this case report.
Kidnie RT: Conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critical revision of the article and final approval of the version to be published.
Sopha SC: Acquisition of photomicrographs and data, critical draft revisions.
Sweet SM: Conception and design, acquisition of data, analysis and interpretation of data, drafting the article, critical revision of the article and final approval of the version to be published.
Author declares that there is no conflict of interest.

©2017 Kidnie, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.