International Journal of
eISSN: 2574-8084


Research Article Volume 3 Issue 1
1Department of Radio-Diagnosis, University of Delhi, India
2Department of Radiotherapy, Delhi Cancer State Institute, India
3Department of Radio-Diagnosis, Delhi Cancer State, India
4Department of Obstetrics and Gynecology, University of Delhi, India
Correspondence: Shuchi Bhatt, University College of Medical Sciences (University of Delhi), & Guru Teg Bahadur Hospital, Dilshad Garden , New Delhi- 95, India, Tel 91-11-22586262, Fax 0091-11-2259049
Received: March 25, 2017 | Published: May 18, 2017
Citation: Bhatt S, Verma P, Grover RK, et al. Success, effectiveness and safety of combined sonographic and fluoroscopic guided percutaneous nephrostomy in malignant ureteral obstruction. Int J Radiol Radiat Ther. 2017;3(1):165-170. DOI: 10.15406/ijrrt.2017.03.00048
Introduction: Percutaneous nephrostomy (PCN) is a palliative procedure required to drain malignant ureteral obstruction to provide reasonable benefit to the patient. The study was conducted with the objective to assess the success, effectiveness and safety of combined USG and Fluoroscopy guided percutaneous nephrostomy in malignant ureteral obstruction.
Method: 69 obstructed ureters due to malignant cause in 50 patients were drained by PCN using combined ultrasound (USG) and fluoroscopic guidance. Post-procedural renal function tests were compared to pre-procedural values using paired t-test. Percentage of correct placement of tube and complications were studied.
Result: PCN was successful in 98.55% units. Significant improvement in renal function occurred in 88% patients. Mean urea dropped from 89.68mg/dl ±39.95 to 65.34±16.55mg/d and mean S. creatinine decreased from 4.31±2.84 to 2.61±1.21 between 14-35days. No statistically significant increase was seen in renal parenchymal thickness. Major complications developed in 4.33% while minor in 50.72% PCN’s with pain being the most common complication in 17.39% cases.
Conclusion: PCN was successfully performed under combined image guidance and it effectively drained the obstructed kidney. It is a safe procedure but has a high minor complication rate leading to patient morbidity.
Keywords: nephrostomy, percutaneous, hydronephrosis, ureteral obstruction, ultrasound, fluoroscopy, kidney
Malignant ureteral obstruction is a urologic emergency and if not relieved, the clinical condition deteriorates rapidly due to increasing uremia and associated electrolyte imbalance finally leading to death.1‒4 Drainage of the obstructed system externally by percutaneous nephrostomy (PCN) or internally by retrograde ureteric stenting provides palliative relief from obstruction which tends to progress despite the treatment of the primary malignancy. PCN is the only available option when the retrograde ureteral stenting is not possible due to anatomical or technical reasons.5,6
Depending on the image guidance, PCN can be an effective and safe procedure but is associated with significant morbidity due to the development of complications.1,7 However, in carefully selected patients it improves the quality of life and prolongs survival as it allows increased acceptance of the palliative treatment because of improved renal functions.
Fluoroscopy is the accepted standard imaging modality for image guided PCN and the success rate is much higher (92%) as compared to ultrasound (USG) alone where it is 83.1%.8 However, peri-organ injury is higher (2.9% Vs. 1.5%) with fluoroscopy and as sonography allows real time imaging it is much safer.8 Combined use of both the modalities is expected to achieve still better results in terms of success rate and decrease in the occurrence of major complications in PCN.9
The final benefit derived from the procedure is largely affected by the success, effectiveness and safety of PCN besides various patient factors. Therefore, it is essential to assess the performance of image guided PCN in successfully and effectively draining the obstructed pelvicalyceal system without causing much morbidity. The study was conducted with the objective to assess the success, effectiveness and safety of combined USG and Fluoroscopy guided PCN in malignant ureteral obstruction.
After obtaining due approval from the ethical review board of both the institutions, this study was conducted in the department of Radio-Diagnosis from November 2013 to March 2015.
Study design
Quasi-experimental study (study undertaken to evaluate the causal effect of an intervention on the target population without the random assignment). Being a quasi-experimental study, the target population was all patients of obstructive uropathy due to a malignant cause and satisfying the selection criteria as outlined below. PCN (using both USG and fluoroscopy guidance) was the intervention to be evaluated by estimating its effect in the form of the procedural success, its effectiveness in relieving the obstruction and the complications incurred during and after the procedure. There was no control group in this study design.
Patient selection
69 obstructed renal units in 50 adult patients due to ureteric involvement by a malignant process or as a result of radiotherapy for urogenital malignancy, when retrograde stenting was not feasible/failed or urgent drainage of the obstructed pelvicalyceal system was required were included in the study. These patients were subjected to image guided PCN after taking an informed written consent. All patients with skin infection at PCN site/septicemia, deranged coagulation profile (INR>1.5, Platelet count<50000), uncooperative and non-consenting patients and terminally ill patients with short life expectancy (ECOG >3) were excluded from the study.
Clinical history, physical examination and relevant laboratory investigations (blood urea, serum creatinine, serum electrolyte, hemogram, urine for culture and sensitivity and coagulation profile) were recorded. 19 patients having low haemoglobin levels and were given blood transfusion to raise the level to 10gm% before undergoing PCN. 14 patients had INR>1.5 and were given fresh frozen plasma. Preliminary USG was done to confirm the presence and side of obstructive uropathy and morphology of the kidney- size, echogenicity, parenchymal thickness, grade of hydronephrosis. The parenchymal thickness was also determined in the post procedure period.
Technique
Patient was admitted prior to the procedure, intravenous access was achieved and prophylactic antibiotics administered as per protocol. Image guidance was provided by ultrasonography (Ultrasound with Digital Color Doppler and Elastography: Acuson Antares (Siemens Medical Ltd) and fluoroscopy (Digital X Ray Fluoroscopy with Flat Panel Detector: Axiom luminous dRF (Siemens Medical Ltd).
Neff percutaneous access set (Cook/Equivalent) was used to access the minimally dilated system while for Moderate/Grossly dilated system, PCN set by Cook/Malecot catheter (Devon/Equivalent) was used.
Patient was placed in the prone position, cleaning and draping of the region was done and skin infiltrated with 1% lignocaine. Following an incision with surgical blade (no 11) puncture needle (18/21 gauge) was introduced into the dilated pelvicalyceal system (PCS) under real time USG guidance using a trans abdominal convex probe of 4-9MHz Figure 1. Urine/pus was aspirated on PCS access for culture and sensitivity.
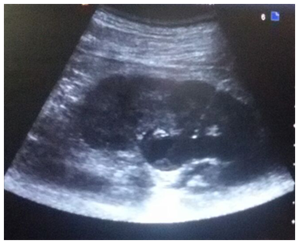
Figure 1 Longitudinal sonogram showing the entire course of the needle(arrow) traversing renal cortex with the tip in the dilated PCS
Precise point of entry was confirmed by a performing a nephrostogram and once the access into target calyx was confirmed the needle was exchanged over 0.035 Terumo guide wire. The tract was serially dilated, catheter introduced and guide removed after confirming correct placement of catheter tip. The nephrostomy tube was secured and urobag attached. Post procedure nephrostogram was performed Figure 2.
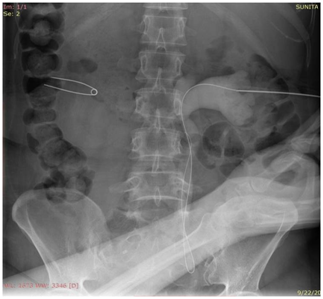
Figure 2b Radiograph showing the guidewire in the opacified PCS and the ureter with the needle in the PCS.
Physiological monitoring of heart rate by ECG, blood pressure and oxygen saturation was done during and after the procedure and patient observed 24hours. Catheter care was advised to the patient and asked to report back if hematuria persisted for more than 48hours. Follow up with blood urea, serum creatinine, and renal ultrasound as done after 2weeks and 1month of the PCN. The routine follow-up continued till end of the study or a terminal event, and outcome recorded.
Technical success was considered to be achieved if the catheter tip was placed in preferred position in the collecting system, with egress of urine/pus from catheter as shown in Table 1. The success was judged by the post PCN nephrostogram. The effectiveness of the PCN was judged by the improvement in the renal function tests in the post procedural period determined after two weeks and then after one month (Table 2) (Table 3). Post-procedural values were not available in 6 patients due to patient demise/lost follow up.
Dependent Variable |
Bd. Urea Mean ± S.D |
S. creatinine Mean ± S.D |
Baseline urea (T1) |
89.68 ±39.952 |
4.31±2.84 |
After 2 weeks (T2) |
70.80±19.786 |
2.97±1.35 |
After one month (T3) |
65.34±16.553 |
2.61±1.20 |
Table 1a Comparision of mean and standard deviation of preprocedural (baseline), and postprocedural (after 2weeks and one month) Blood Urea and S.Creatinine. N=44 patients
Mean Difference |
F value Repeated ANOVA |
|||
Bd. Urea |
S. Creatinine |
Bd. Urea |
S. Creatinine |
|
T1vs T2 |
18.89 |
1.34 |
0 |
0.001 |
T2 vs T3 |
5.45 |
0.359 |
0 |
0 |
T1 vs T3 |
24.35 |
1.699 |
0 |
0 |
Table 1b Comparisons between pre and post urea and ceatinine ( T1 VS T2, T2 VS T3, T1 VS T3)
*F value < .001 is significant.
Mean ± S.D. |
Difference (95% Confidence Interval) |
P value |
|
Pre procedural |
15.20±4.14 |
-.03(-0.15+0.09) |
0.623 |
Post procedural |
15.23±3.97 |
Table 2 Comparison of Preprocedural and Postprocedural parenchymal thickness in 62 units
*p value < .05 is taken as significant.
Timing of Complication |
Complication |
Units for PCN |
|
During Procedure |
|||
·Puncture |
IVC puncture* |
1 |
1.44 |
·Immediate post PCN |
Perinephric haematoma |
2 |
2.89 |
Post PCN |
|||
·Early (within 48 hours) |
Gross haematuria* |
2 |
2.89 |
·Late (more than 48 hours) |
Catheter removal |
5 |
7.24 |
Total |
Minor |
35 |
50.72 |
Table 3 Complications occurring during and after PCN in 69 units ((N=69)) in 50 patients.
*Is a major complication
Baseline and post PCN values of parenchymal thickness was determined sonographically and compared. Parenchymal thickness could not be assessed in 7units in 6patients who were lost to follow up/expired. The various early and late complications were noted and dealt with appropriately, and calculated as percentage per renal units subjected to PCN.
Statistical analysis
Data recorded was analyzed by SPSS-20 software. Success and complication rate was calculated as percentage. For determining the effectiveness of PCN one factor repeated measures ANOVA was used to compare biochemical parameters (Blood urea & Serum creatinine) in baseline and after twoweeks and one month of the procedure. Mauchly’s test of spherecity was applied. Multiple comparisions were made between baseline and post-procedural (after 2weeks and one month) blood urea and serum creatinine using Bonferroni adjustment, F value was calculated and F<0.001 was taken as significant. Paired t test was used to compare parenchymal thickness and p value <0.05 was taken as significant.
There were 19(38%) male and 31(62%) female patients, with male to female ratio of 1:1.63. The patients ranged from 21 to 70years of age with mean age of 57.12years. 22(44%) patients were known cases of malignancy developing ureteric obstruction, while 25(50%) and 3(6%) patients presented with obstructive uropathy and with acute renal failure with simultaneous detection of the malignant pelvic pathology. Carcinoma cervix was the commonest malignancy seen in 48% of patients followed by carcinoma urinary bladder (20%), carcinoma prostate (14%), adenocarcinoma gastrointestinal tract (12%), carcinoma ovary (4%) and carcinoma of endometrial cavity accounting for 2% of cases.
A total of 85 renal units were obstructed, bilateral in 35(70%) and unilateral in 15(30%) patients. Bilateral obstruction was commonest with carcinoma cervix seen in 18 patients (36%). Gross, moderate and mild hydronephrosis was present in 20.28%, 68.56% and 10.13% respectively. The commonest cause was invasion by primary malignancy (48.52%), followed by radiation fibrosis (43.52%) and least with metastatic- peritoneal or ovarian (8.23%) cause.
Image guided PCN was performed in 69 out of 85 obstructed units in 50 patients, bilaterally in 19(38%) and unilaterally in 31(62%) patients. During PCN, 95.42% renal units were successfully punctured in the first while 5.78% in the second attempt. Egress of urine was spontaneous in 91.33% cases and actively aspirated in 8.67% cases, thus in all the cases needle was rightly placed in dilated PCS. Tip of PCN catheter was accurately placed in 68 out of 69units. The technical success rate of PCN was 98.55% (Table 4).
Number of Obstructed Renal Units |
Egress of Urine |
Tip of Catheter |
||||||
Spontaneous |
Active Aspiration |
Within PCS |
Misplaced |
|||||
No. |
% |
No. |
% |
No. |
% |
No. |
% |
|
69 |
63 |
91.33 |
6 |
8.67 |
68 |
98.55 |
1 |
1.45 |
Table 4 Showing egress of urine and position of tip of catheter in 69 obstructed renal units
The pre and post- procedural valves of blood urea and serum creatinine were compared in 44 patients. The mean±2SD levels of blood urea in pre- and post-procedural (at two weeks and one month) period were 89.68±39.952mg/dl, 70.80±19.786mg/dl and 65.34±16.553 respectively (Table 1A) (Table 1B). The mean±2SD levels of serum creatinine in pre- and post-procedural (at two weeks and one month) period were 4.31±2.84mg/dl, 2.97±1.35mg/dl and 2.61±1.20mg/dl respectively (Table 1A) (Table 1B). The pre and post- procedural valves of mean parenchymal thickness were compared in 62 units and determined as 15.20±4.14mm and 15.23±3.97mm respectively (Table 2).
Safety of PCN was assessed by procedure related complications- major (massive hematuria, sepsis, pneumothorax, bowel transgression, colonic perforation, injury to intra-abdominal viscera, or death due to hemorrhage) or minor (microscopic, gross hematuria clearing within 48hours, urine extravasation, perirenal haematoma) and catheter related complication like pain, dislodgement/kinking/blockage/infectious complication/hardening of the tube); occurring during successful/unsuccessful tube placement (Table 3).
Major complication occurred in 4.33% (3 of 69 units), gross haematuria being most common 2.89% cases. Inferior vena cava injury was a rare serious complication encountered in one patient (1.44%), and occurred during tract dilatation. Haematuria continued for 36hours after PCN and the patient died of acute renal shut down (Figure 3). Minor complication rate was 50.72% with persistent pain at PCN site (17.39%) being the most common. On excluding pain, minor complication rate was 33.33%.
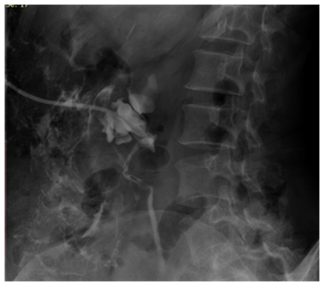
Figure 3 Post PCN nephrostogram showing perinephric extravasation of contrast a complication of the procedure.
44 of 50 patients received palliative treatment either radiotherapy/chemotherapy therapy in the post PCN period, 29(58%) patients expired while 19 patients were alive till end of study and two patients lost to follow up. Though 29 patients had successful urinary diversion, four (8%) died within three days of PCN, 18(35%) had progressive and seven (14%) metastasic disease despite chemo-radiotherapy.
A Quasi-experimental (study undertaken to evaluate the causal effect of an intervention on the target population without the random assignment) study was conducted on image guided (both USG and fluoroscopy) PCN in 69 renal units of malignant ureteral obstruction in 50 patients. Malignancy of pelvic origin is the commonest cause of malignant ureteral obstruction10 as also found in our study with 94% patients. Jose Benito et al.11 found bilateral obstruction in 68.75% patients with pelvic tumours to be the cause whereas tumours from distant sites mostly caused unilateral obstruction11 and these findings were similar to our study.
USG localized the calyx for puncture while fluoroscopy was used for guide wire and catheter manipulations in the present study as also used by other authors.12 In contrast to fluoroscopy real time ultrasound provides excellent cross sectional anatomic detail that allows for access to kidney in a single puncture with confidence.13 Other advantages are avoiding radiation, lesser adjacent visceral/vascular injury. USG also allows for the shortest and most direct access to targeted renal calyx with minimal morbidity.12
Success
In the current study, technical success rate was better than quoted in literature 98.55% which further reiterates the concept of using both USG and fluoroscopic guidance for PCN. Previous studies show a success rate of 90% or more using some guidance modality11,14,15 and 91% to 92%, when USG alone is used for the procedure.14 With improved imaging systems, PCN has largely replaced surgical nephrostomy in drainage of obstructed renal systems and can be safely performed as an outpatient procedure with same day discharge.16
Effectiveness
In our study decrease in blood urea and serum creatinine in post PCN period between 14-35days was statistically significant suggesting an improvement in the functional renal status after PCN was done for malignant ureteral obstruction. This finding is consistent with previous study undergoing palliative urinary diversion for ureteric obstruction in patients having gynaecological malignancy along with symptomatic improvement over a period of 1-3weeks post PCN.17‒19 These studies confirm that urinary diversion can cause rapid restoration of renal function and evasion of the complications of uraemia. PCN also caused lower complication rates therefore, is superior to intraureteral stenting.20
Time of estimation of post procedure renal function tests is an important determinant for renal functional improvement and early testing may not reflect the degree of improvement. Mean of 16.8days are required for serum biochemical values to reach their nadir levels following PCN insertion4 as done in our study. On comparision of the pre and post procedure mean parenchymal thickness no statistically significant improvement (increase) was found.
Safety: complications
PCN is a safe and a minimally invasive procedure. The incidence of major complications (4.33%) was similar to previous quoted studies of 1 to 4-5%.21,22 IVC puncture seen in 1(1.44%) has been reported earlier as well.23,24 Significant haemorrhage warranting transfusion or surgery has been uniformly found to be the most common major complications accounting for 5.3% cases.6 In our study, 2.89% had significant haemorrhage requiring blood transfusion whereas minor bleeding was seen in 8.69% patients which resolved spontaneously with observation and occasional flushing of the catheter.
Previous studies report minor (early and delayed) complication rate ranging from 28-60% excluding pain.6 In our study minor complications rate was 50.72% and excluding pain (17.39%) decreased it to 33.33% suggesting decrease of complications with combined guidance technique. Procedure related complications continue to be widely reported regardless of the type of imaging employed for guidance.6 Rates vary from 25-60% and higher values include late (more than 24hours after PCN) minor complications such as those related to tube malfunction, leakage, dislodgement and incrustations. In our study the procedure related complications were seen in 15.94% PCN and included IVC puncture, perinephric haematoma and haematuria Figure 4. Complications such as infection (10.5%) are also described in literature25 with perinephric abscess rates to be as high as 15%, however in our study no such complication was reported. Complications such as ineffective drainage (12.5%) may require revision secondary to malposition or blockage of the catheter.26,27 Although the percutaneous approach under image guidance has a high technical success rate, long term management of nephrostomy catheters is cumbersome and is associated with an inferior quality of life as compared with internal stents.1 Catheter related complications were commonly encountered (39.13%) in our study also.
All 38% patients alive at the end of the study showed improvement in renal functions and derived benefit of palliative treatment offered to them. Three were successfully converted to DJ stenting after PCN. However, pain at the catheter site also remained the commonest complaint in 12 out of these 19 patients (63.16%) patients.
Limitation of the study was that radionuclide scanning which is a very sensitive means to objectively evaluate renal function was not done in any of our patients. The improvement in individually drained renal unit could not be assessed. Patients undergoing percutaneous nephrostomy were followed only till the end of study and further follow up was not available. PCN is an easy, safe and effective procedure in improving renal functions caused by malignant ureteral obstruction having a high success rate if dual image guidance (USG and fluoroscopy) is used. There is a definite improvement in the renal status which allows time for treatment of primary malignancy to be done.
None.
Author declares that there is no conflict of interest.

©2017 Bhatt, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.