International Journal of
eISSN: 2574-8084


Research Article Volume 1 Issue 1
Division of Radiation Oncology, Medanta Cancer Institute Medanta-The Medicity, India
Correspondence: T Kataria, Division of Radiation Oncology, Medanta Cancer Institute Medanta-The Medicity, Gurgaon, Haryana-122001, India
Received: September 08, 2016 | Published: October 6, 2016
Citation: Kataria T, Goyal S, Malik A, et al. Modulated radiotherapy for breast cancer: locoregional outcomes. Int J Radiol Radiat Ther. 2016;1(1):8-14. DOI: 10.15406/ijrrt.2016.01.00003
Introduction: Breast cancer treatment has evolved considerably over past several decades to enable safe treatment with maximal control and minimum toxicity. Understanding of recurrence patterns with respect to disease stage and biology have enabled fine tuning of radiotherapy (RT) volumes as well as doses. We analysed the data of our breast cancer patients who received local or locoregional radiotherapy with newer techniques to determine recurrence and toxicity patterns
Methods: Treatment records of patients treated with curative intent radiotherapy between November 2009 and November 2013 at division of Radiation Oncology at our institute were analysed. Demographics, treatment details and outcomes were determined from recorded data. Stata software version 9 was used for analysis.
Results: 364 patients with stages I to III was included. Median age was 55years. Most common histology was invasive ductal carcinoma. 68% patients were hormone receptor positive. 22.4% patients had pathologic high risk characteristics. 14.1% pts received neoadjuvant chemotherapy. 48.3% had modified radical mastectomy (MRM), 50.8% had breast conservation therapy while 0.8% had completion mastectomy following lumpectomy elsewhere. Most common RT technique was intensity modulated radiation therapy (74.1%). 3.3% pts had grade 3/4 skin toxicity and 11% had grade 3/4 myelosuppression requiring treatment interruption. There was no difference in toxicity between treatment techniques. After a median follow up of 30 months, there were 39 recurrences (24 distant metastases, 4 locoregional and 4 at both locoregional and distant sites simultaneously). 5 year disease free survival was 86%. Higher T and N stage on pathology and MRM were significantly associated with inferior disease free survival.
Conclusion: Modern RT techniques provide an acceptable locoregional control while minimising toxicities. Omission of axillary radiotherapy in our patients did not increase the risk of locoregional recurrence.
Keyword: intensity modulated radiotherapy, volumetric modulated arc therapy, breast cancer, radiotherapy, chemotherapy, trastuzumab, modified radical mastectomy
Breast cancer is the commonest cancer among females worldwide as well as in India, accounting for 27% of all cancer and 21% of cancer related deaths in India.1 Over last few decades, better understanding of disease biology, early detection and advances in treatment techniques have led to improved outcomes. Newer approaches are being studied to improve treatment outcome with decreased treatment related morbidity.
In India, early breast cancer (EBC) constitutes approximately 30% of all breast cancer cases, in contrast to the West where 60-70% cases have EBC.2 EBC is potentially curable with local treatment but long term toxicities of loco-regional therapy are issues of concern. Surgical techniques have become more conservative as breast conservation surgery (BCS) combined with radiotherapy showed equivalent result as compared to mastectomy in terms of overall survival and local control rates.3,4 Adjuvant radiotherapy reduces the locoregional recurrences by 70% and is indicated in almost all patients undergoing BCS and many patients undergoing modified radical mastectomy (MRM). Despite reduction in local recurrence rates, long term toxicities remain a concern. Furthermore, recent advances in radiation using CT simulator simulator, modern day linear accelerator, computerized treatment planning system modalities and on-board imaging techniques have made it possible to reduce acute and long term toxicity without compromising efficacy. These newer 3-D treatment approaches minimize the radiation dose to lung and heart and hence enable reduction of radiation related complications.5,6 Although the modern techniques of radiotherapy have not shown improvement in survival compared to conventional radiotherapy so far, the reduction in toxicity makes them attractive for regular use.
Sentinel node biopsy has replaced axillary dissection in clinically node negative patients, demonstrating equivalent survival.7,8 Axillary radiotherapy achieves similar control rates with fewer axillary complications compared to surgery in clinically node negative axilla as well as limited sentinel node involvement but axillary dissection remains the standard of care for clinically node positive patients or those with more than two positive sentinel lymph nodes.9,10 Radiotherapy to axilla can be avoided in adequate axillary dissected patients and as well as in patients with low risk axilla or sentinel node negative patients. Moreover, combination of axillary dissection and axilla radiotherapy increases the incidence and severity of lymphadema without adding to therapeutic gain.11 Short term and long term toxicities of axillary dissection can be prevented in sentinel node negative patients and this translates in better quality of life.12
There is paucity of data on the use of modern techniques of radiotherapy in breast cancer from the developing World. In the current study, we have analyzed our experience of adjuvant radiotherapy in patients with early and locally advanced breast cancer at our institution which is a tertiary referral centre in India. Outcomes of local radiation with or without supraclavicular irradiation but no axillary irradiation have been evaluated.
Data source and inclusion criteria
Clinical records of 527 breast cancer patients registered between November 2009 to November 2013 were screened. Patients with metastatic disease, incomplete data, those treated outside (with radiotherapy) were excluded from the final analysis. Finally 364 patients of localized breast cancer who had received adjuvant radiotherapy at our institute were analyzed. Clinical, demographic, pathological details, treatment toxicity and outcome related information was collected from case records and entered in a pre-designed proforma. Disease was re-staged according to AJCC staging system 7th edition base on clinical, radiological and pathological data.
The decision to treat patient with radiation therapy was taken after a review of preoperative clinical findings, relevant investigations including staging work up, kind of surgery performed (BCS versus MRM), intraoperative findings and surgical histopathology in conjunction with the patient's clinical status and other planned treatments (chemotherapy/trastuzumab/hormone therapy) at the time of consultation. Permission for viewing individual case records was obtained from the Institutional Review Board and the medical records department. Confidentiality of the patient's identity was maintained.
Radiotherapy protocol
Simulation: After obtaining informed consent, the patient was taken for mould room procedures, which consisted either of positioning the patient on a breast board or fabrication of a vacuum cushion which helps in reproducible positioning and immobilization. Patients were directed to lie supine with both arms abducted and raised above head. Radioopaque markers identifiable on CT imaging were used to mark the midline, anterior axillary fold (ipsilateral), and upper and lower extent of breast tissue or chest wall on palpation. Postoperative breast or chest wall scars and drain sites, if present, were also marked. External fiducial markers were applied to aid repositioning. With patient in treatment position, CT images were acquired at 3mm slice thickness from the angle of mandible till the lower border of L2 vertebra. For post-mastectomy patients, two scans were acquired: one with a 1cm thick gel bolus covering the chest wall and a second scan without the bolus. Non-ionic intravenous contrast was given at 1.5ml/kg in cases where the target volume was intended to include the supraclavicular nodal region, provided renal function tests was normal.
Volume delineation and dose prescription
The CT scan images were transferred to radiation therapy contouring and planning workstation (MONACO), where delineation of target volumes as per Radiation Therapy Oncology Group (RTOG) guidelines for breast cancer radiotherapy and organs at risk was performed on axial slices by a radiation oncologist.12 Axilla was not part of the target volume in patients who underwent a satisfactory axillary dissection or sentinel node biopsy. Internal mammary chain was sometimes included for medial quadrant tumors with a heavy axillary nodal burden. Supraclavicular region was delineated in cases with locally advanced disease, axillary nodal involvement of 4 or more nodes or with patients who had breast conservation with inner quadrant tumors. The organs at risk included ipsilateral and contralateral lungs, heart, opposite breast, spinal cord, trachea, esophagus. Liver, ipsilateral humerus head and brachial plexus. Patients with EBC were prescribed a dose of 50 gray (Gy) to chest wall or breast at 2Gy per fraction. For patients with locally advanced disease, chest wall was prescribed a dose of 54Gy at 2Gy per fraction. For post-mastectomy patients, the total dose was divided between two plans, the plan developed on CT with bolus delivering 28Gy in 14 fractions, the remaining dose prescribed to the ‘without bolus’ plan. For post-BCS patients, a dose of 50Gy in 25 fractions was prescribed to whole breast with an additional boost of 10-15Gy over 5-6days either immediately after completion of the whole breast radiation or after a gap of 1-2weeks, depending on their tolerance to treatment. Supraclavicular region, when treated, was prescribed a dose of 50Gy in 25 fractions.
Radiotherapy planning
3DCRT Planning: Two tangential semi-opposed beams, physical wedges (15-30 degree), and a multiyear collimator were used. The beam angles and beam weighting were chosen to optimize PTV coverage while minimizing exposure to ipsilateral lung, heart and contra lateral breast. For right-sided disease, gantry angles ranged from 40 to 55 degree for medical field and 220 to 235 degrees for lateral fields; for left-sided disease, gantry angles were 305 to 320 degree for medical fields and 130 to 145 degrees for lateral fields. Leaf margin of 5mm was added in all directions for PTV. Fields were extended 1,5cm beyond the skin anteriorly to provide coverage of the “flash” region. Isocentre was placed at the centre of PTV.
IMRT planning: Seven coplanar intensity modulated equally spaced fields in 200 degree arc around the patient's breast/chest wall were used. The IMRT plan was optimized to cover at least 95% of PTV by 95% of the prescribed dose while minimizing the dose to OARs as much as possible. Inverse planning optimization was performed using Monaco TPS. Dose calculation was done with Monte Carlo semi-biological algorithim with 3 mm grid resolution. Auto flash margin of 1.5cm to PTV was used to extend the fluence outside the body contour. Tissue in homogeneities was considered in the treatment planning optimization process. An optimization with 100 iterations was then applied and followed by a semiautomatic segmentation. Segments with less than ≤2 MUs were expelled from the plan.
VMAT planning: Plans were generated with Monaco TPS in which continuous gantry motion is modeled as a number of discrete angle segments and MLC apertures are progressively added throughout optimization. MUS per gantry angle were optimized using a variable dose rate. Gantry incremental angle was kept at 10 degree. Monte Carlo dose calculation algorithm was used with heterogeneity corrections and grid size of 3mm. Auto flash margin of 1.5cm to PTV was used to extend the fluence outside the body contour. One coplanar semi arc with gantry angle between 290 degree to 160 degree in clockwise rotation was used for planning and delivery in left-sided disease and between 200 degree to 80 degree in right-sided lesions. The collimator angle was set to 0 degree.
Plan evaluation and statistical analysis: Dose volume histograms (DVH) were used to evaluate all plans. Plan was approved once it was found satisfactory with respect to coverage of PTV and avoidance of organ at risk according to our institutional criteria. Attempts were made to keep both the plans similar in coverage, conformity and homogeneity. DVHs were used for evaluation and comparisons of dose to OARs.
Figures 1A & Figure 1B show the interplan comparison of beam arrangement and dose distribution for two representative patients.
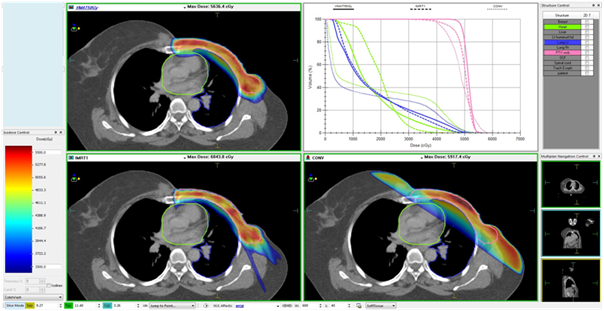
Figure 1A Carcinoma left breast, post-mastectomy, T3N0M0, planned for adjuvant RT to left chest wall (50Gy in 25 fractions). Patient was treated with 5-field IMRT plan. VMAT and tangential plans were made secondarily for comparison.
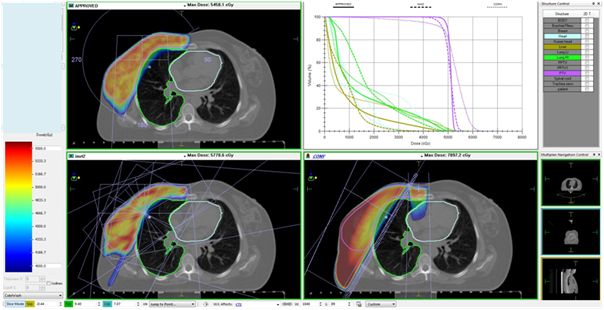
Figure 1B Carcinoma right breast, post breast conservation surgery T2N0M0, planned for adjuvant RT to right breast (50Gy in 25 fractions). Patient was treated with VMAT. 5-field IMRT and Tangential plans were made for comparative assessment. Subsequently tumor bed boost of 12.5Gy in 5 fractions was delivered using 12MeV electrons prescribed at 90% isodose.
Verification and treatment delivery
After verification of treatment set up with cone beam CT images, necessary corrections were applied before treatment delivery. Patients were reviewed at least once a week for morbidity assessment and CTC version 3 was used for scoring toxicity. Weekly complete blood counts were undertaken for all patients during the course of radiotherapy.
Follow up
Patents were considered on continuous follow up if the last visit was within 3months of censoring the data. In cases where last visit was more than 3 months earlier, attempts were made to telephonically contact the patients. Patients were followed from the date of registration to the date of death or last visit (if lost to follow up) and censored at the date they were last known to be alive or 15th March 2015, whichever was earlier.
Statistical methods
The data was censored on 15th March 2015 or last follow up date (if lost to follow up). Descriptive statistics was used for describing demographic and clinical characteristics of the patients. Fisher Exact test was used to assess correlation between variables. Survival estimation was done by Kaplan-Meier method. Cox regression analysis was performed to evaluate impact of disease characteristics on recurrence free survival. Overall survival was defined as duration from registration till death due to any cause and recurrence free survival was defined as duration from registration till recurrence of disease or death. Analysis was done using the Stata sofware (Release 9.0, Stata Corp).
A total of 364 patients with localized disease (stage I-III) were included in the analysis. Median age was 55years (range 31-87years). Invasive ductal carcinoma was the most common histology found in 351(96.36 %) patients. Status of estrogen receptor (ER), progesterone receptor (PR) and Her-2/neu receptor was available for 359, 357 and 346 patients, respectively. Of these, 245 patients (68.25%) were ER positive, 218(61.06%) were PR positive and 90(26.01%) patients were Her-2/neu receptor positive by IHC and/or FISH. Detailed pathology information was available for 361 patients, on which 81 patients (22.44%) had high risk features.
Neoadjuvant chemotherapy was given in 51(14.01%) patients. Surgical techniques consisted of MRM in 176(48.35%) patients, BCS in 185(50.83%) and completion mastectomy after incomplete surgery done elsewhere in 3(0.82%) patients. Stage distribution and other clinical and demographic details are summarized in Table 1.
Characteristics |
N =364 |
% |
Age |
Median 55.5 yrs (31-87) |
|
Sex |
364 |
|
Males |
1 |
|
Females |
363 |
|
Grade |
256 |
|
Grd 1 |
31 |
12.11% |
Grd 2 |
143 |
55.86% |
Grd 3 |
82 |
32.03% |
High risk features in histopathology |
361 |
|
Absent |
280 |
77.56% |
Present |
81 |
22.44% |
Estrogen receptor |
359 |
|
Positive |
245 |
68.25% |
Negative |
114 |
31.75% |
Progesterone receptor |
357 |
|
Positive |
218 |
61.06% |
Negative |
139 |
38.94% |
Her-2-neu |
346 |
|
Positive |
90 |
26.01% |
Negative |
256 |
73.99% |
Stage |
360 |
|
DCIS |
1 |
0.28% |
Stage Ia |
55 |
15.28% |
Stage Ib |
3 |
0.83% |
Stage Iia |
101 |
28.06% |
Stage Iib |
72 |
20.00% |
Stage IIIa |
71 |
19.72% |
Stage IIIb |
14 |
3.89% |
Stage IIIc |
43 |
11.94% |
Type of surgery |
364 |
|
BCS |
185 |
50.83% |
MRM |
176 |
48.35% |
Completion mastectomy |
3 |
0.82% |
RT technique used |
364 |
|
IMRT |
269 |
73.90% |
VMAT |
81 |
22.25% |
Others |
14 |
3.85% |
Table 1 Baseline characteristics and locoregional treatment received
Intensity Modulated Radiotherapy (IMRT); volumetric modulated arc therapy (VMAT)
For adjuvant radiotherapy, intensity modulated radiotherapy (IMRT) was used in 269(73.9%) patients, volumetric modulated arc therapy (VMAT) was used in 81(22.25%) patient and 13(3.85%) patients were treated with conventional 3D conformal radiotherapy.
Skin toxicities were grade 2 or less in most patients (351 patients, 96.43%). Thirteen patients (3.57%) had grade 3 skin toxicity. No grade 4 toxicity was reported. Myelosuppression grade 3-4 requiring treatment interruption was seen in 40(10.9%) patients. Mild dysphagia (grade 2) requiring symptomatic treatment was observed in 78 (21.43%) patients. Only 1 patient had radiological evidence of lung toxicity while three patients had self-limiting cough only without evidence of radiation pneumonitis on imaging. There was no statistically significant difference in toxicities among the three radiation techniques used (Table 2).
Skin toxicity |
RT technique |
||
IMRT |
VMAT |
Others |
|
Grd 0 |
(3.35%) |
3(3.70%) |
1(7.14%) |
Grd 1 |
164(60.96%) |
57(70.37%) |
9(64.29%) |
Grd 2 |
87(32.34%) |
17(20.99%) |
4(28.57%) |
Grd 3 |
9(3.35%) |
4(4.94%) |
0 |
Grd 4 |
0 |
0 |
0 |
Total |
269 |
81 |
14 |
P=0.49 |
|
|
|
Table 2 Radiation therapy Technique Vs acute skin toxicity
Intensity modulated radiotherapy (IMRT); volumetric modulated arc therapy (VMAT)
After a median follow up time of 30months, there were 34 recurrences, of which 24 were distant metastases, three were locoregional recurrences alone and seven patients had both local as well as distant recurrences. Four patients failed locally; of which only 1 was isolated recurrence, 1 in combination with supraclavicular region and 2 with distant metastases. Three patients failed in the axilla but only 1 of these was an isolated axillary recurrence, remaining 2 in combination with distant metastases. There were 3 supraclavicular recurrences but supraclavicular region was never the only site of relapse. All local recurrences and supraclavicular recurrences were in-field, that is, the recurrence occurred within the PTV. Axilla was never part of PTV. In the only case where there was an isolated axillary recurrence, the patient had stage I (T1N0M0) disease. Disease free survival at 2, 3 and 5years was 91.75%, 88% and 86%, respectively. On Univariate Cox regression analysis, higher pathological T stage (pT3 and pT4), higher nodal stage (pN2 and pN3) and conservative surgery (BCS) were significantly associated with inferior disease free survival. Type of radiotherapy technique used did not impact disease free survival.
Variable |
N |
HR |
95% CI |
P |
Age |
||||
≤60 |
238 |
1 |
||
>60 |
124 |
1.04 |
0.51-3.04 |
0.89 |
Pathological high risk feature |
||||
Absent |
280 |
1 |
||
Present |
81 |
1.37 |
0.61-3.04 |
0.44 |
Grade |
||||
1 |
31 |
1 |
||
2 |
143 |
1.31 |
0.15-10.88 |
0.8 |
3 |
82 |
4.17 |
0.53-32.31 |
0.17 |
pT stage |
||||
pT 0,1 and 2 |
304 |
1 |
||
pT 3 and 4 |
55 |
2.47 |
1.18-5.17 |
0.01 |
pN stage |
||||
pN0 and 1 |
257 |
1 |
||
pN2 and 3 |
105 |
2.31 |
1.17-4.54 |
0.015 |
Type of surgery |
||||
Mastectomy (MRM/ completion) |
188 |
1 |
||
BCS |
176 |
3.22 |
1.49-7.14 |
0.003 |
RT technique used |
||||
IMRT |
269 |
1 |
||
VMAT |
81 |
0.81 |
0.34-2.07 |
0.72 |
Others |
12 |
0.73 |
0.09-5.4 |
0.75 |
Table 3 Univariate cox regression analysis for disease free survival
The last two decades have witnessed several developments in the field of imaging and radiation therapy planning and delivery techniques. Improvements in radiotherapy technology have allowed us to deliver effective treatments with good cosmesis without the added burden of long term complications such as fibrosis and cardiac toxicity. CT simulators, modern linear accelerators, three-dimensional (3D) planning techniques and treatment verification modalities provide improved targeting of regions of interest while maximally sparing normal tissues. Several retrospective and few prospective randomized studies have demonstrated that IMRT improves the dose homogeneity and decreases the incidence and severity of toxicity after BCS in EBC.13‒15 Among the earliest studies, Kestin et al from William Beaumont Hospital showed dosimetric superiority of IMRT over standard tangents as well as uniformity of doses. In their preliminary data, there was minimal skin toxicity with IMRT across various breast sizes.13 In addition, field matching is superior when irradiating chest wall/breast along with nodal basins.14 Vicini et al demonstrated similar treatment times as 3DCRT, thus advocating widespread use of IMRT without compromising clinic resources and time constraints.15 As far as the difference in efficacy is concerned, there is only one randomized study comparing IMRT and non-IMRT. Mukesh et al randomized 815 patients to either standard wedge-based tangential fields or forward planned IMRT. They found no statistically significant difference in 5year locoregional recurrence rates (2.56% vs 1.35%) or overall survival (92.5% vs 91.7%).16 Additionally, two studies have evaluated breast cancer related outcomes, the retrospective study by McDonald et al and the prospective sutdy by Mc Donald MW et al.,17 Morganti et al.18 None of these studies reported a statistically significant difference in survival, disease-specific survival or freedom from contralateral breast cancer recurrence between IMRT and non-IMRT techniques. These data have established the role of IMRT as a non-inferior technique with respect to the oncologic outcomes while solidifying its superiority in terms of adverse effects, specifically skin toxicity owing to better dose homegeneity in IMRT plans compared to 3DCRT or tangential field plans.
The major benefit with the use of modern techniques of radiotherapy is reduction in early and late toxicities. Pignol et al, in their multicentre study on 358 patients comparing standard tangents with IMRT, demonstrated significant reduction in the rate of moist desquamation with IMRT (31.2% vs 47,8%, p=0.002). Moist desquamation was significantly correlated with increased pain and reduced quality of life.19 Harsolia et al have demonstrated the lower rate of grade 2 or 3 dermatitis with IMRT as compared to conventional wedge based 2 D RT (41% vs 85%, p<0.001).20 Similarly McDonald et al have shown that IMRT results in lesser grade 2-3 dermatitis as compared to conventional 3DCRT.17 These results have been consistent in other studies as well. In our study, we did not note a significant difference in skin toxicity between the various techniques; however, the proportion of patients who were treated with tangents or 3DCRT was small and a meaningful comparison could not have been made.
Axillary irradiation has increasingly been discouraged in regular practice, especially following an adequate axillary nodal dissection due to associated high risk of debilitating lymphedema. Our departmental protocol does not include routine irradiation of axilla even in patients with heavy axillary burden if axillary dissection or sampling is adequate. Our results support the belief that omitting axillary irradiation does not compromise oncologic outcomes. It is possible that in some cases, incidental irradiation to axilla might have taken care of micrometastases as demonstrated by our group in an earlier publication.21 Even when axilla was not a part of target volume, nearly 50% dose was delivered to the axilla in the IMRT plans, and may have been sufficient to address the microscopic disease in a small proportion of patients.
In our cohort of patients, higher tumor and nodal stage were associated with inferior outcomes. This is in concordance with the published literature. Additionally, our observation of lower disease free survival in mastectomy patients compared to breast conservation may have been a reflection of case selection bias wherein bigger tumors or those with clinical nodal involvement were more often favored for mastectomy. Almost half of our patients have undergone BCS. In contrast, other series from India have reported lower rates of BCS.22,23 This difference may be due to selection bias of the retrospective study, as we have included only those patients who have undergone adjuvant radiotherapy. The low rate of axillary recurrence overall (0.82%) and axilla as the sole site of recurrence (0.27%) in our study reemphasizes the practice of avoiding axillary radiation in low axillary risk group without axillary dissection and high risk with complete axillary dissection.
The patient cohort in this study was not uniformly segregated between the treatment modalities, which show the process of gradual evolution and implementation of newer techniques with resultant decreased use of 3DCRT in comparison with IMRT and increasing experience and confidence gained with VMAT. This was a limitation in terms of inability to quantify differences in toxicities between treatment techniques but there is enough historic evidence to prove the dosimetric and clinical superiority of IMRT over conventional treatment techniques (Figure 2).
the use of modern radiotherapy techniques in adjuvant setting in early breast cancer results in fewer acute toxicities and gives comparable survival outcomes. However, complexities of planning and infrastructure required for the same are challenges for their wide applicability, particularly in a country like India.
None.
Author declares that there is no conflict of interest.

©2016 Kataria, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.