International Journal of
eISSN: 2574-8084


Research Article Volume 6 Issue 1
1Immanuel Kant Baltic Federal University, Kaliningrad, Russia
2Infectious Diseases Hospital of Kaliningrad Region, Kaliningrad, Russia
Correspondence: VA Izranov, Immanuel Kant Baltic Federal University, Kaliningrad, Russia
Received: January 29, 2019 | Published: February 22, 2019
Citation: Izranov VA, Stepanyan IA, Kazantseva NV, et al. 2D-shear wave elastography and laboratory non-invasive indices in the diagnosis of liver fibrosis. Int J Radiol Radiat Ther. 2019;6(1):42-45. DOI: 10.15406/ijrrt.2019.06.00211
The evaluation of the stage and extent of liver fibrosis is crucial for the prognosis and management of patients with chronic liver diseases. There are frequent late requests for the medical help in the development of cirrhosis and its complications due to the long-term asymptomatic course of liver fibrosis. Determination of fibrosis at an early stage allows for timely therapy and stopping or slowing the progression of fibrosis to the final stage of decompensated cirrhosis.
Aim: The aim of the study is to analyze the morphological and physiological changes in the liver and identify the most informative non-invasive laboratory method for determining liver fibrosis.
Materials and methods: It was analyzed 3 groups of patients (n=102) with chronic hepatitis B and C and liver cirrhosis of viral etiology. For all patients, de Ritis coefficient, Aspartateaminotransferase to Platelet Ratio Index (APRI) and the test «Firosis-4» (FIB-4) were calculated and an ultrasound examination of the abdominal organs was carried out with the determination of the sizes of both liver lobes and 2D-SWE elastometry of the liver using the SuperSonic Imagine’s Aixplorer (France).
Results: Among the laboratory non-invasive diagnostic methods the most effective was the index APRI. 2D-SWE elastometry allows to determine the minimal and moderate fibrous changes in the liver parenchyma. It is revealed that the left liver lobe increases already at the minimum fibrous changes. The integrated use of APRI and FIB-4 indices with 2D-SWE elastometry in patients with chronic viral hepatitis is a non-invasive alternative to diagnosing liver fibrosis and cirrhosis and may reduce the need for liver biopsies.
Keywords: diffuse liver diseases, non-invasive diagnostic methods, radiology, ultrasound, biochemical indexes
Diagnosis and treatment of chronic diffuse liver diseases represent one of the current interests of modern hepatology due to the high prevalence of the pathology, significant deterioration of the quality of life of patients and an increase in mortality from complications. According to the World Health Organization (WHO) statistics, mortality from cirrhosis and liver cancer has increased in the world in the last years: in 2000, the cause of death from cirrhosis ranked 13th, in 2017-11th; mortality from liver cancer in 2000-20th place, in 2017-16th place.
Liver fibrosis is a key element in the development of the pathological process in the liver tissue,1 is the result of chronic damage to the liver tissue and excessive accumulation of extracellular matrix proteins, developed under the influence of various etiological factors: viral and autoimmune hepatitis, toxic effects, metabolic disorders (fatty hepatosis, amyloidosis, hemochromatosis), congenital and acquired immunodeficiency states, hematological diseases, chronic heart failure. The prognosis and management of patients with chronic liver diseases of any etiology is largely determined by the severity and length of fibrosis, which is a kind of measure of the activity, prevalence and duration of pathological changes in the liver.2
The main pathway for the progression of chronic diffuse liver diseases is the development of successive stages of fibrosis with the formation of eventually cirrhosis and an increased risk of developing hepatocellular carcinoma.3 Liver fibrosis is asymptomatic for a long time and often patients seek medical help only with the development of cirrhosis and its complications.2
Diagnosing fibrosis at an early stage allows for timely therapy and stopping or slowing the progression of fibrosis to the final stage of decompensated cirrhosis.4 In this regard, various methods of non-invasive diagnosis of liver fibrosis, which have no contraindications and complications, have become widely introduced into medical practice, in contrast to the liver biopsy, which is the "gold standard" in the diagnosis of fibrosis.
On the other hand, interpretation of the results of the study of such transaminases as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the blood serum by way of a mandatory screening test is becoming increasingly important.5 In most cases, ALT and AST are targeting markers of damage and necrosis of hepatocytes.6
In practice, clinicians, in addition to direct transaminases values, also take into account the AST/ALT ratio, called the De Ritis coefficient. Normally, its value is 1.3±0.4. The significance of the De Ritis coefficient in serum was repeatedly studied in various pathological processes of the liver. The analysis of modern publications demonstrates that in the De Ritis coefficient value in uncomplicated viral hepatitis or non-alcoholic liver damage does not exceed 1 or decreases to 0.6–0.8; an increase of it more than 1.4 is observed in severe alcoholic and toxic liver lesions with destruction of the main mass of hepatocytes and in cirrhosis, or in other nosologies (myocardial infarction, myocarditis, cardiomyopathy, accumulation diseases, for example, in Wilson-Konovalov disease, the coefficient may exceed 4).7
The ratio of aspartate aminotransferase to platelet count (APRI, Aspartateaminotransferase to Platelet Ratio Index; calculated by the formula: AST×100/upper limit of AST×platelet level) was proposed as a non-invasive and readily available tool for assessing liver fibrosis in chronic hepatitis B and C.8
There is also an inexpensive and non-invasive test called FIB-4 (Fibrosis-4), which includes standard biochemical values (ALT, AST), platelet count as an indicator of the fibrosis severity, and the patient’s age9 and is calculated by formula: age (in years)×AST/platelet level×√ALT.
Throughout the history of medicine, palpation of tissues has been an important diagnostic method. Hardness/stiffness of the parenchyma are a key property of pathologically altered tissues. On palpation, the tumor structure is felt as a stiffer tissue compared to the surrounding tissues.10 In the last decades, diagnostic methods have appeared that allow visualization of tissue stiffness—elastography and elastometry methods—the development of “ultrasonic palpation”.
The stiffness of the liver correlates with the degree of fibrosis and indirectly with portal hypertension, that is, with fibrotic changes in the liver, the rigidity of its tissue diffusely increases (elasticity decreases).11 Elastographic evaluation of liver stiffness is a replacement marker for liver fibrosis, which in recent years has been increasingly introduced for non-invasive evaluation and staging of fibrosis in patients with chronic liver diseases.12
When conducting a two-dimensional shear wave elastography (Supersonic shear wave-elastography, 2D-SWE), tissues shift at different depths due to multiple focused acoustic impulses. The 2D-SWE is based on the determination of the speed of propagation of a transverse shear wave-a quantitative estimate of the stiffness of the liver. Shear waves are transverse in contrast to longitudinal ultrasonic waves and propagate more slowly in the soft tissues. The higher the shear wave velocity, the greater the stiffness of the liver tissues. Based on the obtained results, a color map is formed in the window of interest, which is superimposed on the usual gray-scale information (B-mode)-a qualitative assessment of tissue rigidity (with a change in color from blue at F0 to red at F4 degree of fibrosis). 2D-SWE elastometry can be performed many times due to the safety and non-invasiveness of the study.
The aim of the study is to analyze the morphophysiological changes in the liver and to identify the most informative non-invasive routine method for determining liver fibrosis in 3 groups of patients (chronic hepatitis B and C and liver cirrhosis of viral etiology).
Objectives
Comparative performance between the liver fibrosis stage in different liver diseases according to the conventional METAVIR scale obtained during 2D-SWE elastometry with aminotransferase values (ALT and AST), de Rytis coefficient, calculated liver fibrosis indexes (APRI, FIB-4) and liver size.
After obtaining informed consent, there was analyzed 102 patient histories of the Infectious Diseases Hospital of the Kaliningrad Region with chronic hepatitis B (14 patients), chronic hepatitis C (56 patients), and liver cirrhosis of viral etiology (32 patients). The age of patients ranged from 2 to 73 years.
Based on laboratory tests (complete blood count, biochemical blood test), non-invasive markers of liver fibrosis were calculated for all patients: de Ritis coefficient, APRI, and FIB-4. All the studied patients underwent an ultrasound study of the abdominal cavity with the determination of the both liver lobes size and 2D-SWE elastometry of the liver using the SuperSonicImagine's Aixplorer apparatus (France) with measurement of the parenchyma stiffness and determination of the fibrosis stage using the conventional METAVIR scale from F0 to F4 (Figure 1).
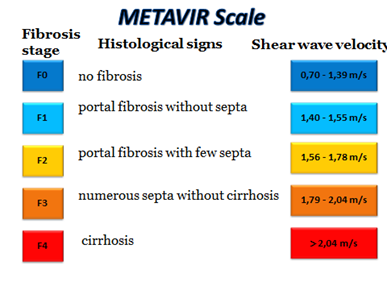
Figure 1 Fibrosis stages according to the conventional METAVIR scale, histological evaluation of fibrosis and the corresponding shear wave velocity.
2D-SWE elastometry of the liver was carried out in the position of patients on the back with the right upper limb set aside for the head in the state of breath-holding or with quiet breathing, the convex sensor was installed in the VII, VIII, IX intercostal space along the anterior axillar and/or medial axillary lines. From 9 to 12 measurements of the shear wave velocity (in m/s) in 5-6 segments of the liver at a depth of 2-6cm from the liver capsule were performed in each patient, on the basis of which the mean and median values were calculated.13–17 The allowed interquartile range (IQR) was no more than 30%.
Liver size is a significant indicator of the presence of a pathological process. Hepatomegaly is a frequent reaction of the liver to the pathological process. In our study, it was found that with diffuse liver lesions, the size of the left lobe first increases (from F0-F1 stage), and the size of the right lobe exceeds the limits of the norm in the later stages of fibrosis (from F2).
The differences reliability between patient groups was statistically significant (p≤0.05), but the de Rytis coefficient was not outside the normal range. According to our data, the APRI and FIB-4 indices are more informative, and their increase is noticeable in the analysis of the total sample (Table 1).
|
Non-invasive indexes |
Normative range |
Mean value |
Standard deviation, SD |
|
De Ritis coefficient |
0,91 – 1,75 |
1,083 |
0,051 |
|
APRI |
0 – 1 |
2,516 |
0,045 |
|
FIB-4 |
0 – 3,25 |
3,69 |
0,04 |
Table 1 Values of noninvasive indexes in common sample of patients
Detection of normal transaminase activity does not exclude the presence of severe fibrosis. Consequently, the severity of liver damage must be assessed by additionally to transaminase activity methods. The de-Ritis coefficient decreased with increasing fibrosis (less than 1), and increased with cirrhosis (more than 1), which is indicated pronounced destructive changes in the hepatic parenchyma.
In patients with hepatitis B, with an increase in the degree of liver fibrosis, the level of the APRI index doubled at the stage of liver fibrosis F1-F2 on the METAVIR scale (Figure 2).
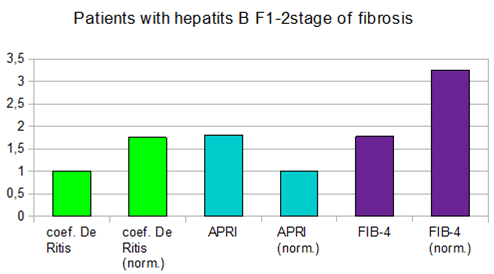
Figure 2 Values of de-Ritis coefficient, APRI and FIB-4 indices at the F1-F2 stages of liver fibrosis (by METAVIR scale) in patients with hepatitis B.
In patients with hepatitis C, the indices of APRI and FIB-4 increased simultaneously, starting to exceed the norm at F3 on the METAVIR scale (Figure 3), at F4 the indices exceed the normal value twice (Figure 4).
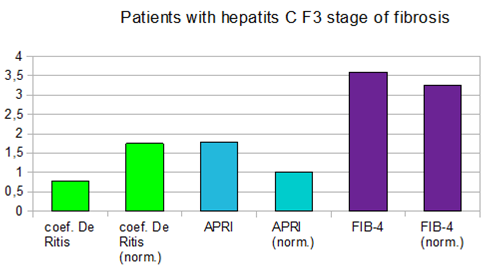
Figure 3 De-Ritis coefficient values, APRI and FIB-4 indices at the F3 stage of liver fibrosis (by METAVIR scale) in patients with hepatitis C.
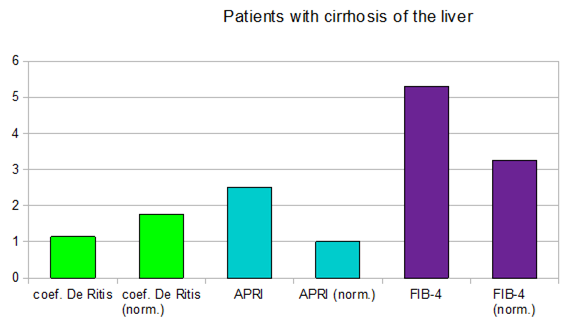
Figure 4 De-Ritis coefficient, APRI and FIB-4 index values at the F4 stage of liver fibrosis (by METAVIR scale) in patients with hepatitis C.
In patients with diagnosed cirrhosis and F4 stage of fibrosis, according to the conventional METAVIR scale, the results of the APRI and FIB-4 indices significantly exceeded the norm (Figure 5).
Thus, the integrated use of the APRI and FIB-4 indices with 2D-SWE in patients with chronic viral hepatitis makes it possible to accurately determine the severity of pathological changes, including the early stages of fibrosis (F1-F2), is a non-invasive alternative to diagnosing fibrosis and liver cirrhosis and may reduce the need for liver biopsies. These methods make it possible to perform screening, conduct dynamic monitoring, assessing the effectiveness of treatment, the rate of disease progression.
None.
The author declares that there is no conflicts of interest.

©2019 Izranov, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.