International Journal of
eISSN: 2573-2889


Case Report Volume 2 Issue 2
1Vocational School of Health Services, Adiyaman University, Turkey
2Department of Medical Biology and Genetics, Turkey
3Department of General Surgery, Adiyaman University, Turkey
4Department of Pathology, Faculty of Medicine, Çukurova University, Turkey
Correspondence: Osman Demirhan, Department of Medical Biology and Genetics, Faculty of Medicine, Çukurova University, 01330 Balcalı-Adana, Turkey, Tel 90-322-3387140, Fax 90-322-3386572
Received: February 17, 2017 | Published: March 3, 2017
Citation: Korkmaz DT, Demirhan O, Çetinel N, et al. Sex chromosome alterations in undifferentiated pleomorphic sarcoma. Int J Mol Biol Open Access. 2017;2(2):47-51. DOI: 10.15406/ijmboa.2017.02.00014
The X and Y chromosomes have been associated with malignancy in different types of human tumors. However, the function of sex chromosome aneuploidies (SCAs) is not yet fully known, it would be interesting to understand what the SCAs may be doing in sarcoma tissue.
The aim of this study: It was to evaluate, the numerical aberrations of chromosomes X and Y in a patient with undifferentiated pleomorphic sarcoma (UPS), using fluorescence in situ hybridization (FISH) and by cytogenetic techniques.
Materials and Methods: A patient with UPS underwent. The FISH technique was used to sarcoma tissue ;furthermore, standard technique for cultivation of lymphocytes from peripheral blood of the patient was used for identification of Y and X chromosomes.
Results: A total 433 cancer cells were analyzed FISH. SCAs were found in 28.4%(123 cells) of 433 cancer tissue-cells (please explain – SCAs were found in 28.4% of all cells or numerically in 123?). In the blood, the SCAs were found in the 5.6% of 250 cells analyzed (how many cells were analyzed?). 72.4% and 27.6% of 123 cells with SCAs had the addition and loss of one or more extra X or Y chromosomes in cancer tissue, respectively. There was a significant difference in the frequencies of the increase (123 SCAs ) and loss of sex chromosomes, and the increase in sex chromosomes was higher in the tumor tissue (p<0.005). More specifically, polysomies were observed in 72.4% of the cells with SCAs. The other common karyotype was the loss of one Y (LOY), which was observed in 26.8% of the cells with SCAs. LOY were observed to be most frequent in blood of the patient (64.3%).
Conclusion: Our results may suggest that the genetic instability associated with sex chromosome polysomies and LOY may account for the considerable potential for risk of sarcoma tumors.
Keywords: fısh, pleomorphic sarcoma, sex chromosome alterations
Cytogenetic analysis of human solid tumor cells is complex, and progress has been slow. However, the karyotypes of some solid tumors can be utilized in analytical investigations of the etiology, diagnosis, and prognosis of cancers. Cytogenetic studies of soft tissue sarcomas (STSs) in humans have remained largely unexplored. Undifferentiated pleomorphic sarcoma (UPS) is a well-known tumor that usually involves the soft tissues, and accounts for less than 5% of STSs in adults.1 STSs in general are rare tumors occounting for less than 1% of all cancers.2,3 UPS is the most common STS seen in patients over the age of 40. Chromosomal abnormalities (CAs) are one of the hallmarks of neoplastic cells in general, and the persistent presence of chromosome instability has been demonstrated in many human cancers. Aneuploidy in human cells is considered to be age and cancer-related.4,5 SCAs are characterized by the addition or loss of one or more X or Y chromosomes. Increased cancer risks have been reported in association with particular SCAs in breast cancer.6 Loss of sex chromosome is the largest source of aneuploidy,7 and efforts have been made to better understand its role in these processes. The observation prompted a critical review of two soft tissue sarcomas in which it similar changes in the X chromosome were identified.8 The loss or gain of an X chromosome in women with aging is much more frequent than that of the Y chromosome in males.9 Some researchers suggested that, although the loss of one Y (LOY) should be recognized as a common age-related event, the percentage of LOY is not dependent on age or disease.4,10 The United Kingdom Cancer Cytogenetics Group concluded that LOY in elderly males was not indicative of malignancy and should not be interpreted as a tumor marker.11 The association of LOY with hematological cancers has been more elusive. More recently, it was demonstrated that the frequency of LOY was significantly higher in patients with hematological disorders than in patients without such disorders, indicating that LOY is associated with a neoplastic process.12 However, paradoxically, X chromosome aneuploidy is rarely seen in the dividing cells of the bone marrow in females.13 A LOY is frequently observed in myeloproliferative diseases and myelodysplastic syndromes, and can also be seen in lymphoproliferative disorders such as lymphomas.14 A LOY in contrast, is a common secondary change in both cancer cells and in leukemias.15 Do sex chromosome aneuploidies play any role in carcinogenesis? To our knowledge, this is the first study carried out to describe the role of SCAs in the pathogenesis of sarcoma cancer, using FISH and standard cytogenetic techniques.
We report a 73-year-old male with UPS . The tumor presented clinically as an enlarging mass in the left breast with a history of one year. There was no history of trauma, nipple discharge, or any other breast lumps. Furthermore, patient had no personal or family history of breast cancer and has never received prior chemotherapy or radiotherapy. The patient had no history of blood transfusion. On physical examination, in the left breast a firm and rounded mass, about 7x7cm diameter, behind the nipple areola complex was palpable. There was no retraction of the overlaying skin and no palpable lymph nodes in the left axillary. Right breast and right axillary were completely normal. Breast ultrasound revealed a solid hypoechoic mass with central necrosiss in the left breast, bilateral axilla were reported as normal. A computer tomography indicated 5x8cm mass with high peripheral vascularity and hypodense necrotic central appearance (Figure 1). In the lower lobes of the left lung, two nodules were observed in the superior and medial basal segments, measuring 7mm and 6mm respectively (Figure 1). There was no pectoral muscle invasion by the mass. Tru-cut biopsy confirmed the pathological diagnosis of UPS and wide excision including nipple areola copmlex was performed (Figure 2). Postoperative course went uneventful. Written informed consent was obtained from the patient. Tissue samples of the primary tumor were taken by the general surgery department. A small piece of the tumor sample and the peripheral blood sample were obtained for genetic study (for FISH and chromosome analysis). The analysises of the FISH and chromosome in cancer and blood samples were performed/done in the genetic laboratory of the Department of Medical Biology and Genetics, Faculty of Medicine, Çukurova University. Standard cytogenetic techniques to detect the blood SCAs were used.
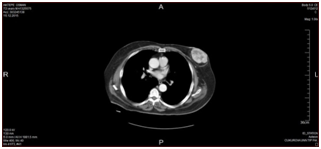
Figure 1 A computed tomography scans of the mass: Normal bilateral axillathe tumor mass in the left breast with high peripheral vascularity and hypodense necrotic center.
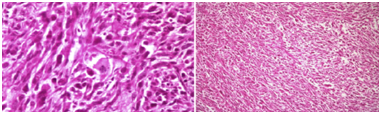
Figure 2 Pathologicalimages of undifferentiated pleomorphic sarcoma (own images of the patient or taken from a publication – clarify and citation if necessary).
All tumor samples were mechanically minced and enzymatically disaggregated by digestion with trypsin‑EDTA (Biological Industries Israel Beit‑Haemek Ltd.) for 1h. The presence of one chromosome Y(green signal) and one X chromosome (red signal) in the nuclei identified male cells (Figure 3). Sex chromosomes were detected in cancer and blood tissues of case as single cells or in cluster. Standard cytogenetic techniques were used for harvesting and preparation of slides for FISH, and 433 metaphases were analyzed. The FISH technique, with centromeric-specific DNA probes, allows for the rapid detection of numerical chromosomal aberrations of tumor cells. All samples were mechanically minced and enzymatically disaggregated by digestion with trypsin‑EDTA (Biological Industries Israel Beit‑Haemek Ltd.) for 1h. Therefore, interphase FISH was used to study X and Y chromosomes in non‑dividing cells. Identification of SCAs in tumor tissues. To further characterize the data obtained by a FISH was performed neoplastic tissues from man. The presence of one chromosome Y (green signal) and one X chromosome (red signal) in the nuclei identified male cells. Sex chromosomes were detected in case, as single cells or in cluster. Standard cytogenetic techniques were used for harvesting and preparation of slides for FISH, and 433 metaphases were analyzed. Standard technique for the cultivation of lymphocytes from peripheral blood of the patient was used for identification of Y and X chromosomes and the preparations were treated with trypsin to obtain G-banding. Evaluation of karyotypes was done according to ISCN (2005) standards, and 250 metaphases were analyzed (Figure 3).
A total of 433 UPS cancer cells were analyzed using FISH. 71.6% (310 cells) of cancer cells had normal karyotype. SCAs were found in 28.4%(123 cells). In the blood, 94.4% of 250 cells had normal karyotype. 14cells or 5,6% were identified with SCAs. . The frequencies of SCAs were higher in the tumoral tissues than in the blood (28.4 % vs 5.6%), and this difference was found statistically significant (p<0.0001). The frequencies and distributions of X and Y chromosome aneuploidies detected are shown in (Table 1).
Karyotypes |
Cancer Tissue |
Blood Tissue |
||
Number % |
Number % |
|||
Normal Karyotype (XY) |
310 |
71.6 |
236 |
94.4 |
Sex Chromosome Aneuploidies |
123 |
28.4 |
14 |
5.6 |
Total |
433 |
250 |
||
Sex Chromosomal Abnormalities |
||||
XXYY |
39 |
31.7 |
- |
|
X (-Y) |
33 |
26.8 |
9 |
64.3 |
XXY |
15 |
12.2 |
2 |
14.3 |
XXYYYY |
15 |
12.2 |
- |
|
XXYYY |
11 |
8.9 |
- |
|
XYY |
2 |
1.6 |
2 |
14.3 |
XYYY |
2 |
1.6 |
- |
|
Y (-X) |
1 |
0.8 |
1 |
7.1 |
The others |
5 |
4.1 |
- |
Table 1 Characteristics of the sex chromosomes
In the UPS cancer tissue, SCAs were identified in 28.4% of cells. The 72.4% of SCAs had the addition one or more extra X and Y, and 27.6% had loss of one X and Y. There was a significant difference in the frequencies of the addition or loss of sex chromosomes addition of sex chromosomes was higher in the tumor tissue (p<0.005) than in the blood (Table 1). Specifically, numerical additions of chromosomes Y and X, including polysomies, were observed in 72.4% of the cells The sex chromosome trisomies were present in 13.8% of SCAs, including 47,XXY (12.2%) and 47,XYY(1.6%). Numerical aberrations of chromosome Y and X, including polysomies were observed in 54.4% of the SCAs (XXYY, XXYYYY, XXYYY, XYYY). The most common polysomie seen among chromosome increases was the XXYY chromosomes (31.7%). The other common karyotype was LOY, which was observed in 26.8% of the cells with SCAs. Specifically, LOY were the most frequent in the blood of the patient (64.3%);loss of chromosomes X was observed in only one blood cell (7.1%), indicating that the LOY was more frequently seen in the blood than the loss of X. The sex chromosome trisomies were present in 28.6% of SCAs, including 47,XXY (14.3%) and 47,XYY (14.3%) (Table 1).
The statistical analysis was performed using the statistical package for social sciences (SPSS/PC 19 version, 2010). In the statistical analysis, the Fischer’s exact test was used to determine the significance of the differences between the blood and tissues of the patient in terms of the number of SCAs. The differences were considered significant at p<0,05.
Most human cancers display structural and numerical CAs, and there is a growing evidence that at least some of these aberrations play an important role in the development of all cancer types.16 Cytogenetic studies of soft tissue sarcomas have remained largely underexplored, and the significance of SCAs in soft-tissue sarcomas has not been clearly established yet. To date nonrandom primary changes have been found in two types of soft tissue sarcoma - liposarcomas17 and certain subtypes of rhabdomyosarcoma.18,19 Another soft tissue sarcoma, synovial sarcoma, has been analyzed extensively in the clinical and pathology literature, but its chromosome pattern has been described in only one single report.20 Aneuploidies are a commonly observed feature in some cancers, and it has been suggested that is a driving force in tumor development by enhancing genomic instability. In our case, 28.4% of the UPS tumor cells and 5.6% of the peripheral blood cells had SCAs. The frequencies of SCAs were higher in the tumoral tissue than in the blood (p<0.0001). Why SCAs appear to be present at higher levels in STS? These findings show that there is an association between malignancy and SCAs. w is this conclusion drawn as the patient had already a tumor – how do you connect the risk to develop malignancy with these findings?
The unique biology of X-Y homologous regions has attracted considerable interest because of the identification of new tumor suppressor genes whose alterations may play a role in the development and progression of human tumors. The observation prompted a critical review of two soft tissue sarcomas in which a study had identified similar changes in the X chromosome.8 At the same time, X chromosome was found to be involved in carcinogenesis and the malignant progression of different types of human tumors, and an increasing number of potentially responsible genes have been identified.21 In the present study, chromosomes Y and X polysomies in the UPS were more than aneuploidies . Specifically, numerical increases of chromosomes Y and X, including polysomies (XYY, XXY, XXYY, XYYY, XXYYY and XXYYYY), were the most frequent (72.4%). With regard to polysomy Y in relation to the clinical and histopathological data of bladder cancer patients,22 to our knowledge, there are no detailed reports.
In a study, high polysomies of certain chromosomes were found in this group of tumors.23 Loss of X chromosome is very rare. However, several studies have shown that the X-chromosome additions were found to be involved in carcinogenesis and the malignant progression of different types of tumors, an increasing number of potentially responsible genes have been identified. case reports have suggested the possibility of the associations of X polysomy with several malignancies.24,25 Polysomy X has been reported in transitional cell carcinoma cell lines.26 The addition of one X chromosome is also relatively common in leukemias, lymphomas, and prostate cancer, and generally occurs in association with other karyotypic changes.27,28 It is really not known whether this addition involves the active or the inactive X chromosome. Although there are numerous X linked genes that may be involved in neoplasia, including the MAGE tumor-specific antigen loci, the pseudoautosomal GM-CSFR gene possibly escapes X chromosome inactivation29,31 (finish or revise the sentence). In the present study, specifically the most common karyotype seen among the Y and X polysomies was the 48,XXYY (31.7%). It thus appears that a gene(s) raising the risk of UPS is located on the X or Y chromosomes, and is overexpressed because it escapes the X inactivation; both in males and females with extra X chromosomes.32 X-chromosome inactivation has slowed our understanding of the involvement of this chromosome in cancer, since X chromosomes that are involved in aneuploidies and rearrangements may be either active or inactive. It appears that the numerical increases of the X and Y chromosomes in UPS may be preferentially involved and important in detecting cancer development.
In the present study, loss of X and Y chromosomes were observed the 71.4% in blood tissue and the 27.6% in cancer tissue. In particular, the LOY were found in 64.3% of blood tissue and in 26.8% of cancer tissue. The UPS cells had a higher incidence of aneuploidies, and specifically LOY were observed to be the second most frequent. Genes involved in cancer development and progression have often been identified by their loss (for tumor suppressor genes) or activation (for oncogenes) as a result of chromosomal rearrangements. LOY is a frequently reported chromosomal abnormality in many tumor types. The question arises of whether there is a primary neoplastic event which precipitated the LOY or loss of X.33,34 The cause and relevance of the LOY remain unclear, because of the associations between the LOY and the clinical outcome. Later studies have supported the theory that the LOY is a nonphenotypic event associated with the aging process in males.10,35 However, other studies have shown that age is not clearly related and the X and Y chromosomes that are lost reappeared after therapy and during clinical remission. Therefore, it supports the hypothesis that the loss of sex chromosome (in the cancer tissueis due to the evolution of a malignant clone. A missing Y was reported as a frequent event in bladder transitional cell carcinoma, which can occur either early in tumor progression or at advanced stages of the disease. Previous studies have shown that there is a relationship between the LOY and bladder cancer (BC) in males.36 The applied for LOY has been determined in 10–40% of BC.37 However, the clinical significance of the LOY is largely unknown, since relatively few sets of male BC patients have been evaluated. At the same time, LOY is frequently observed in myeloproliferative diseases, myelodysplastic syndromes and ANLL and can also be seen in lymphoproliferative disorders like lymphomas. Also, the LOY was a common secondary change in cancer cells and in some leukemias, and common in many tumor types including papillary renal cell cancer.38
We found that the most common genetic changes in our case of undifferentiated pleomorphic sarcoma tissue were Y and X polysomies, and LOY were the second most frequent. Our findings suggest that the Y and X aneusomies play a role in the pathogenesis of UPS, and may be further studied as a surrogate marker for detection of cancer development/tumor progression, undifferentiated pleomorphic sarcoma cancer.
None.
Author declares that there is no conflict of interest.

©2017 Korkmaz, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.