International Journal of
eISSN: 2573-2889


Research Article Volume 8 Issue 1
1Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Nigeria
10Department of Microbiology, Faculty of Life Sciences, University of Ilorin, Nigeria
11National Biotechnology Research and Development Agency, Nigeria
12Department of Food Science and Technology, Faculty of Agriculture, Nnamdi Azikiwe University Awka, Nigeria
13Department of Chemical Engineering, School of Infrastructure and Processing Engineering Technology, Federal University of Technology, Nigeria
14Department of Biological and Environmental Sciences, Liverpool John Moores University, Liverpool, United Kingdom
2Department of Microbiology, Faculty of Science, Federal University of Lafia, Nigeria
3Department of Medical Biochemistry, Faculty of Basic Medical Sciences, University of Ilorin, Nigeria
4Department of Biochemistry, Faculty of Biological Sciences, University of Nigeria, Nigeria
5Department of Biochemistry, Faculty of Basic Medical Sciences, University of Lagos, Nigeria
6Department of Nutritional, Food and Consumer Sciences, Fulda University of Applied Sciences, Germany
7Department of Human Nutrition and Dietetics, Faculty of Public Health, University of Ibadan, Nigeria
8Department of Chemistry, College of Physical Sciences, Federal University of Agriculture, Nigeria
9Department of Medical Biochemistry, College of Health Sciences Nile University of Nigeria, Nigeria
Correspondence: Moses Adondua Abah, Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Taraba State, Nigeria
Received: December 28, 2024 | Published: January 8, 2025
Citation: Mayel M, Abah MA, Ayo GF, et al. Production of glucose oxidase from Aspergillus Niger using yam peels as carbon source. Int J Mol Biol Open Access. 2025;8(1):1-6. DOI: 10.15406/ijmboa.2025.08.00190
Glucose oxidase is a versatile enzyme with a wide range of practical applications in industries such as food and beverages, healthcare, and biotechnology. As the demand for glucose oxidase continues to increase in diverse sectors such as food processing, pharmaceuticals, and biofuel production, finding sustainable and efficient methods for large-scale production and purification of this enzyme from fungi, particularly Aspergillus niger is paramount. This study was carried out in order to isolate and produce glucose oxidase from yam peels as carbon source. In this study, the microorganism was isolated from Aspergillus niger using yam peels as carbon source. Soil samples were collected randomly from a sugar cane market, at the bagasse dumping site, Donga Local Government, Taraba State Nigeria, whereas yam peels were also collected randomly from Block D female hostel in Federal University Wukari Campus, Wukari, Taraba State Nigeria, in the month of October, 2023. A.niger was isolated from the soil samples and screened for the production of glucose oxidase. Substrate concentration, pH optimization, temperature optimization studies were carried for glucose oxidase The kinetic parameters for glucose oxidase were also studied. The effect of incubation days and protein concentration on glucose oxidase production and activity respectively were also investigated. The results obtained from this study showed that optimal conditions for glucose oxidase production appear to be at day 5 of fermentation (2.375 μmolmin-1), with peak activity observed at a temperature of approximately 40°C (0.831 μmolmin-1) and a pH range of 5-6 (0.312 and 0.283 μmolmin-1, respectively). The kinetic parameters (Vmax and Km) for glucose oxidase produced were found to be 5.0 mmol/min and 0.254 mM respectively. The study concludes that glucose oxidase can be produced from A. niger using yam peels aas carbon source and findings from this study also revealed the necessity for careful optimization of fermentation conditions in industrial processes utilizing glucose oxidase. The study recommended that further investigation should be done on the molecular mechanisms underlying the effects of temperature and pH on glucose oxidase activity.
Keywords: glucose oxidase, isolation, yam peel, aspergillus niger, concentration, substrate
Glucose oxidase (GOX) has found widespread application in various sectors, including the food and beverage industry, clinical diagnostics, biofuel production, and the textile industry.1 In the food industry, glucose oxidase is utilized for the improvement of the texture and shelf life of products, removal of glucose or oxygen from food products (e.g., during the manufacture of egg powder), production of gluconic acid and determination of D-glucose in biological fluids such as blood and fermentation products.1,2 In clinical diagnostics, it plays a vital role in glucose monitoring. They can also be used as a mild acidulant in metal, leather and for preventing browning during dehydration caused by the Maillard reaction. Moreover, its application in biofuel production and the textile industry underscores its potential to contribute to sustainable technologies and environmentally friendly processes. Glucose oxidase is an oxido-reductase with an EC number (1.1.3.4). This enzyme catalyzes the oxidation of β-D-glucose to D-glucono-1, 5-lactone and hydrogen peroxide using molecular oxygen as the electron acceptor.3 Glucose ovidase is highly specific for the β-anomer of D-glucose, while the α-anomer does not appear to be a suitable substrate. Low GOX activities are exhibited when utilizing 2-deoxy-D-glucose, D-mannose and D-galactose as substrates. Inhibitors of GOX include p-chloromecuribenzoate, Ag+, Hg2+, Cu2+, hydroxylamine, hydrazine, phenylhydrazine, dimedone and sodium bisulphate. On average, the isoelectric point of GOX has been shown to fall between pH 4 and pH 5.4
Glucose oxidase has been purified from a range of different fungal sources,5,6 and particularly Aspergillus and Penicillium species, have been extensively explored.5,7 These fungi are known to produce glucose oxidase as a part of their metabolic processes, and this natural abundance makes them a promising source for enzyme isolation. The most common fungal sources of the enzyme are Aspergillus niger, Penicillium notatum, Penicillium glaucum, Penicillium amagasakiense, and Penicilliumpurpurogenum.4,8,9 Bacterial species also have the capacity to produce glucose oxidase such as Gluconobacter oxydans, Zymomonas mobilis. Acetobactor methanolicus, Micrococcus, and Enterobacter.10 However, Fungi are considered to be of more valuable in the enzyme production than the bacteria. Fungal species are important in the fields of microbiology and biochemistry due to their ability to produce useful enzymes. Fungal enzymes have been employed for the rapid oxidation and decomposition of proteins, carbohydrates and fats.11 Enzyme production by fungi is commonly performed by solid-state fermentation, submerged fermentation or surface-adhesion fermentation.12–14 However, biotechnological processes with immobilized growing fungi cells, including those for extracellular enzyme production, seem to be more favorable than traditional fermentation methods since immobilization enables repetitive and continuous use of the microbial cells.12,15 As the demand for glucose oxidase continues to increase in diverse sectors such as food processing, pharmaceuticals, and biofuel production, finding sustainable and efficient methods for large-scale production and purification of this enzyme from fungi, particularly Aspergillus niger, is paramount. There is need to increase and enhance GOX production and performance in order to broaden its applicability in point-of-care diagnostics, continuous glucose monitoring systems, and various biotechnological applications. This research aimed at producing glucose oxidase from a local fungal isolate, Aspergillus niger using yam peel as carbon source. This study could provide insight on GOX enzymatic activity and also contribute to proper understanding of fungal biology and their implication for bioprocess engineering and biotechnology.
Sample collection
Soil samples were collected randomly from a sugar cane market, at the bagasse dumping site, Donga Local Government, Taraba State Nigeria, during the month of October, 2023.Yam peels were also collected randomly from Block D female hostel in Federal University Wukari Campus, Wukari, Taraba State Nigeria, in the month of October, 2023.
Isolation of fungiAgar (PDA agar) was prepared by weighing 3.9 g of powdered Agar. This was dissolved in 100 ml of distilled water under flame and autoclaved at 121oC for 5 minutes after which it was kept in the dark and allowed to cool. It was then sealed with foil paper. 7 test tubes were placed on a test tube rack filled with 9 ml of distilled water. 1g of the soil sample was weighed and transferred to the first tube and saturated from the first tube. 1ml of the solution was transferred to the test tubes. This process was repeated until the last tube was 10ml.4 plates were selected and 0.1ml of the diluted sample was placed in the plate and covered care was taken in the stage as the plates were labeled with the name of the dilutions (plate 1; 10-1, plate 2; 10-2, plate 3; 10-3 and so on). 0.1ml of soil dilution of 10-1 was poured into the plate labeled 10-1 and 10ml of soil dilution of 10-2. This was due until the 7th plate containing 10-6 dilution solution was covered immediately. About 15-20 ml of Agar was poured into the plates labeled 10-1 to 10-6 and swirled gently to avoid splash at the surface of the plates. The plate was supplemented with Streptomycin to avert bacterial contaminations. Finally, the plate was allowed to solidify then inverted and incubated for 24 hours at 39oC.
Subculture of enzyme-producing microorganismAgar solution was prepared by weighing 3.9 g of the Agar pounder and dissolved in 100 ml of distilled water under flame, autoclaved at 121°C for 15 minutes after which it was kept on the desk and allowed to cool. It was sealed with a foil paper (autoclaving was done using conical flask). The Agar solution was contained in a conical flask before introducing to autoclaving chamber). After the Agar has cooled, it was poured into 6 plates still labeled with (10-1 to 10-6) and allowed to solidity. Following the observation from the phase experiment, it was discovered that plate 2 (10-1), 4 (10-3) and 6 (10-5) had a good colony condition among others. Plate 2 (10-1), 4 (10-3) and 6 (10-5) was picked using a sterile wire loop and a burner. Culture plate 1 was inoculated into the new prepared medium same for plate 2 and plate 4 to obtain the pure culture for these organisms. The new medium with the inoculate organisms was inverted at 37oC for growth after 24-48 hours.14
Screening of fungal isolatesAgar solution was prepared by weighing 3.9g of the Agar powder and dissolving it in 100ml of distilled water in a conical flask. This was autoclaved at 121oC for 15 mins after which it was kept on the desk, allowed to cool and sealed with a foil paper. 1% O-dianisidine and 1% peroxidase were prepared. 15-20ml of the Agar medium were then poured and 100ml of peroxidase and 100ml of o-dianisidine was added and incubated after 48hrs. A brown colored zone around the medium was observed while the colony grew only on the middle of the plate.
Fermentation for glucose oxidase productionThe fermentation medium for the production of glucose oxidase was containing the following chemicals. Glucose, peptone, (NH4)2HPO4, KH2PO4, MgSO4.7H2O and CaCO3.14 The preparation of the medium was done in percentages for the second batch. 3 conical flasks containing the basal medium were prepared. 8% Glucose, 0.3% peptone, 0.038% (NH4)2HPO4, 0.0188% KH2PO4, 0.0156% MgSO4.7H2O, 3.5% CaCO3 was required in 100ml of distilled water since the nutrient source are expressed in percentages and a conical flask was needed for the carbon source. A total of 24g of Glucose, 0.9g peptone, 0.1164g of (NH4)2HPO4, 0.0564g of KH2PO4, 0.0468g of MgSO4.7H2O and 10.5g of CaCO3 were weighed into a conical flask and a total of 300ml of distilled water was used to dissolve the chemical nutrients. This produced a milky coloration. 50ml of the basal medium was poured into a conical flask and 30g of yam peel was added to the conical flask to make it moist. The conical flask was autoclaved at 121oC for 15 mins to remove contaminations after which a loopful of A. niger was inoculated into the conical flask and tightly sealed and allowed to ferment for 5 days.12
Isolation of glucose oxidaseA sterile medium size spoon was used to scoop a desired fermented medium from the flask and distilled water was used to filter the mass to obtain at least 20ml of the filtrate which contain the crude enzyme. The enzyme was extracted using citrate phosphate.0.1ml citrate phosphate was needed and since commercially prepared citrate phosphate buffer was acquired, 10ml of the buffer was diluted with 100ml of distilled water since the commercial buffer was 1m concentration. Extraction was done before filtration. After filtration, the crude extract was centrifuged at 4000rpm for 10 mins to obtain the supernatant. This contain the enzyme and was stored in EDTA bottle. Fermentation assay for peak production day was carried out by Faizyme method.12 The following was prepared. Peroxidase solution was prepared by weighing 1g of peroxidase and dissolving in 100ml of distilled water. 1g of o-dianisidine was dissolved in 100ml of distilled water. 10% Glucose was prepared by weighing 10g of Glucose in 100ml of distilled water after which a set of cleaned test tube was used. 2.4ml of o-dianisidine + 0.5ml of Glucose + 0.1ml of peroxidase + 0.1ml of enzyme and was allowed to stand for 20 mins after which the absorbance reading was taken at 436nm. This was repeated for 8 days.
Determination of protein concentration
Determination of protein concentration was carried out using the method of Timothy.15 2% sodium carbonate was prepared by dissolving the 2g of sodium carbonate weighed in 100ml of distilled water. 0.1m sodium hydroxide was prepared by weighing 4g of sodium hydroxide in 100ml of distilled water. 2% sodium carbonate and 0.1m sodium hydroxide were mixed together. This mixture formed reagent A. 0.5% Copper sulphate was prepared by dissolving 0.5g of the Copper sulphate chemical in 100ml of distilled water. 1% potassium sodium tartarate was prepared by dissolving 1g of the chemical in 100ml of distilled water. 0.5% Copper sulphate and 1% potassium sodium tartarate were mixed together to give reagent B. Reagent C was prepared by mixing 50ml of reagent A and 1ml of reagent B. Reagent D was prepared by mixing equal volume of 0.1m NaOH with folin-Ciocalteu reagent. Protein concentration was then determined by the following. 1ml of Bovine serum Albumin + 5ml of reagent C + 0.2ml of enzyme, then incubated at room temperature for 10 minutes after which 0.5ml of reagent D was added and incubated at dark cupboard for 30 minutes. After this process, the absorbance for protein content was determined at 660nm.This procedure was repeated for 8 days to determine the protein concentration.
pH optimizationThis was done using 3 buffers namely: sodium phosphate buffer, sodium acetate buffer and tris HCl buffer.0.1m sodium phosphate buffer was prepared by diluting 10ml of the commercial buffer in 100ml of distilled water.0.1m sodium acetate buffer was prepared by diluting 10ml of the commercial buffer in 100ml of distilled water.0.1m tris HCl buffer was prepared by diluting 10ml of the commercial buffer in 100ml of distilled water. pH optimization was carried out in six test tubes each having 2.0ml of GOX enzyme and 1.0ml of glucose then using a pH meter, the pH of the test tube was adjusted to 4, 5, 6, 7, 8 and 9 using HCl and NaOH. Then the activity is determined at 510nm.
Temperature optimizationThe optimization of temperature was done using 8 test tubes, 8% glucose solution. In each test tube, 2ml of distilled water and 1ml of glucose solution was transferred, then 2ml of the GOX enzyme after which one test tube was kept at room temperature (37oC). The next at ice temperature (4oC) and the other 6 test tubes, water bath was used to equilibrate its temperatures at 30, 35, 40, 45, 50, 55 degrees Celsius respectively. After 5 minutes, the activity was taken at 510nm.
Substrate concentration optimizationThis was done using different glucose concentrations in 12 test tubes as described below. These 12 test tubes contained glucose of different concentrations (2mls each of the glucose concentration in the test tube). Then 3ml of distilled water and 2ml of enzyme were transferred into all test tubes and the absorbance was read at 510nm for activity.
Test tube 1: Glucose 4% = 4g in 100ml distilled water
Test tube 2: Glucose 5% = 5g in 100ml distilled water
Test tube 3: Glucose 6% = 6g in 100ml distilled water
Test tube 4: Glucose 7% = 7g in 100ml distilled water
Test tube 5: Glucose 8% = 8g in 100ml distilled water
Test tube 6: Glucose 9% = 9g in 100ml distilled water
Test tube 7: Glucose 10% = 10g in 100ml distilled water
Test tube 8: Glucose 11% = 11g in 100ml distilled water
Test tube 9: Glucose 12% = 12g in 100ml distilled water
Test tube 10: Glucose 13% = 13g in 100ml distilled water
Test tube 11: Glucose 14% = 14g in 100ml distilled water
Test tube 12: Glucose 15% = 15g in 100ml distilled water
Glucose oxidase kinetic parametersThe substrate specificity of purified glucose oxidase was tested on D-glucose at concentrations ranging from 1-10 mmol/L. A Lineweaver-Burke plot was generated by plotting 1/(v) (enzyme activity) vs 1/(S) (substrate concentration). The plot was used to calculate the Km and Vmax values, where Km is the substrate concentration at which the enzyme activity is half its maximum value, whereas Vmax is the maximum velocity of the enzyme reaction.
The ability of some fungal isolates to produce glucose oxidase was tested. The screening result shows that the primary screened fungal isolates upon inoculation at room temperature showed the appearance of a clear zone which is an indication of glucose oxidase production The result revealed lightly some morphological characteristics of A. niger (Figure 1).
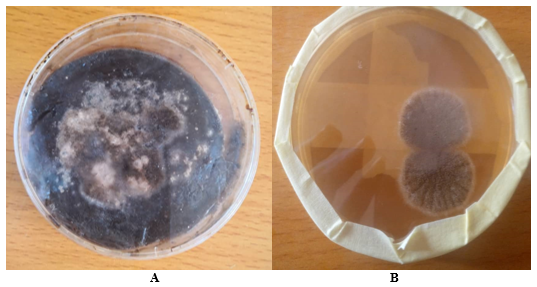
Figure 1 Screened of fungal isolates.
(A) Morphological appearance of fungal growth (B) Screened fungal plate showing the appearance of a clear zone
Fermentation for glucose oxidase production
Table 1 and Figure 2 presents the result of production of Glucose Oxidase from submerged fermentation. The selected isolates from the primary screening method were subjected to solid-state fermentation in a suitable medium and observed. The highest enzyme activity was recorded on day 5 of fermentation. The isolates were found to be able to utilize the available carbon source to produce glucose oxidase. Glucose oxidase production increased gradually over 24 hours of incubation until optimum glucose oxidase production was achieved on day 5. After the optimum incubation period, glucose oxidase production began to decrease with the least production of glucose oxidase recorded on day 8.
|
Days of incubation |
Activity (μmolmin-1) |
|
Day 1 |
1.587 |
|
Day 2 |
1.644 |
|
Day 3 |
1.660 |
|
Day 4 |
1.809 |
|
Day 5 |
2.375 |
|
Day 6 |
0.093 |
|
Day 7 |
0.081 |
|
Day 8 |
0.063 |
Table 1 Production of Glucose Oxidase from submerged fermentation
Table 2 and Figure 3 reveals the result of protein concentration during the incubation period for Glucose Oxidase. The result showed that the highest protein concentration was obtained at day 2 of incubation. Meanwhile, the least protein concentration was achieved at day 3 incubation period for Glucose Oxidase. A sharp decrease in the concentration of protein was noticed after it peaked at day 2 of incubation. Protein concentration began to increase again after day 3. At day 5 of incubation however, decrease in the concentration of protein was noticed until day 8.
|
Days |
Protein concentration (U/g) |
|
Day1 |
0.42 |
|
Day 2 |
0.46 |
|
Day 3 |
0.09 |
|
Day 4 |
0.270 |
|
Day 5 |
0.296 |
|
Day 6 |
0.188 |
Table 2 Protein concentration during incubation period for Glucose Oxidase
The result of the effect of substrate concentration on Glucose Oxidase activity is presented in Table 3 and Figure 4 below. The result revealed that glucose oxidase activity was found to be optimum at 14% and 15% substrate concentration whereas, glucose oxidase showed the least activity at 5%. Substrate concentration. Increase in substrate concentration gave a commensurate increase in enzyme activity. However, beyond substrate concentration 10%, glucose oxidase activity began to decrease but began to increase again beyond substrate concentration 10%.
|
Substrate Concentration (%) |
Activity (μmolmin-1) |
|
5 |
2.144 |
|
6 |
2.161 |
|
7 |
2.170 |
|
8 |
2.173 |
|
9 |
2.173 |
|
10 |
2.179 |
|
11 |
2.177 |
|
12 |
2.174 |
|
13 |
2.176 |
|
14 |
2.184 |
|
15 |
2.184 |
Table 3 Effect of substrate concentration on Glucose Oxidase activity
The result of the effect of pH on glucose oxidase activity is captured in Table 4 and Figure 5 below. The optimum pH for glucose oxidase activity was found to be pH 5 while the lowest activity exhibited by glucose oxidase was at was at pH 4. Beyond pH 5, glucose oxidase activity was seen to decrease until it got to pH 8.
|
pH |
Activity (μmolmin-1) |
|
4 |
0.249 |
|
5 |
0.312 |
|
6 |
0.283 |
|
7 |
0.271 |
|
8 |
0.268 |
Table 4 Effect of pH on Glucose Oxidase activity
The result of the effect of temperature on glucose oxidase activity is captured in Table 5 and Figure 6 below. The optimum temperature for glucose oxidase activity was found to be 40oC Glucose oxidase exhibits its activity the least at 35oC.
|
Temperature (oC) |
Activity (μmolmin-1) |
|
4 |
0.726 |
|
30 |
0.758 |
|
35 |
0.105 |
|
37 |
0.735 |
|
40 |
0.831 |
|
45 |
0.726 |
|
50 |
0.772 |
|
55 |
0.753 |
Table 5 Effect of temperature on Glucose Oxidase activity
In this study, the kinetic parameters of glucose oxidase were investigated. The kinetic parameters (Vmax and Km) for glucose oxidase produced found to be 5.0 mmol/min and 0.254 mM respectively. The result also showed that as the reciprocal of enzyme activity decreased, the reciprocal of substrate concentration also decreased Table 6 and Figure 7.
|
1/V |
1/S |
|
0.466 |
0.2 |
|
0.463 |
0.167 |
|
0.461 |
0.143 |
|
0.46 |
0.125 |
|
0.46 |
0.111 |
|
0.459 |
0.100 |
|
0.459 |
0.091 |
|
0.46 |
0.083 |
|
0.46 |
0.077 |
|
0.458 |
0.071 |
|
0.458 |
0.067 |
Table 6 Kinetic data for Glucose Oxidase
Glucose oxidase is a versatile enzyme with a wide range of practical applications in industries such as food and beverages, healthcare, and biotechnology. As the demand for glucose oxidase continues to increase in diverse sectors such as food processing, pharmaceuticals, and biofuel production, finding sustainable and efficient methods for large-scale production and purification of this enzyme from fungi, particularly Aspergillus niger, is paramount.1 The present study investigated the production of glucose oxidase from submerged fermentation. Change in incubation days during the production of glucose oxidase from submerged fermentation varied such that the activity of 1.587, 1.644, 1.660, 1.809, 2.375, 0.093, 0.081 and 0.063 μmolmin-1 were recorded on day 1, 2, 3, 4, 5, 6, 7 and 8, respectively. The highest enzyme activity was recorded on day 5 of fermentation followed by day 4. Least activity was observed on day 8. During the initial days of fermentation, the enzyme activity steadily increased, indicating active microbial growth and the synthesis of glucose oxidase. The subsequent peak in activity on day 5 suggests that the culture reached its maximum capacity for enzyme production. Beyond this point, factors such as nutrient depletion or accumulation of inhibitory metabolites may start to impede further enzyme synthesis, leading to a decline in activity.15
This study also investigated the concentration of protein during the incubation period for glucose oxidase. The concentration of protein during the incubation period for glucose oxidase gave 0.42, 0.46, 0.09, 0.270, 0.296 and 0.188 U/g on day 1, 2, 3, 4, 5, 6, 7 and 8, respectively. On day 2, the highest concentration of protein was observed followed by day 1. This early peak in protein synthesis may reflect the initial phase of microbial growth and enzyme production, where the culture rapidly synthesizes proteins to meet metabolic demands. The least concentration of protein during the incubation of glucose oxidase was observed on day 6. The decrease in protein concentration observed on day 6 suggests that factors other than protein concentration may be influencing enzyme activity at this stage.
In our study, it was observed that at substrate concentrations of 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 and 15%, glucose oxidase showed activity of 2.144, 2.161, 2.170, 2.173, 2.173, 2.179, 2.177, 2.174, 2.176, 2.184 and 2.184 μmolmin-1 respectively. The data indicates that glucose oxidase activity exhibits a trend of increasing activity with increasing substrate concentrations, reaching a peak at 14% and 15%. This suggests that higher concentration of glucose provided more substrate for the enzyme to act upon, resulting in enhanced enzymatic activity. It was also observed that at lower substrate concentration like 5%, the data showed a decrease in glucose oxidase activity. This phenomenon can be attributed to the limited availability of substrate molecules for the enzyme to catalyze, resulting in reduced enzymatic activity. The trend observed in this study is in line with the concept of substrate saturation by Michaelis-Menten kinetics which states that as substrate concentration increases, the rate of enzyme-catalyzed reactions also increases until a point of saturation is reached.14 However, further increase in substrate concentration may not significantly affect the rate of reaction, as the enzyme becomes saturated with substrate molecules.
For pH optimization studies, glucose oxidase activity was observed to be 0.249, 0.312, 0.283, 0.271 and 0.268 μmolmin-1 for pH 4, 5, 6, 7 and 8 respectively. The data reveals a clear trend, with maximum glucose oxidase activity observed at pH 5, followed by pH 6, 7, and 8, while the lowest activity is recorded at pH 4. This suggests that there is an optimal pH range for glucose oxidase to exhibit its activity. The lower glucose oxidase activity observed at pH 4 could be attributed to the acidophilic nature of the fungi involved in the fermentation process. Fungi typically thrive in acidic environments, but excessively low pH values may adversely affect their cellular metabolism, hence, production of glucose oxidase. Our finding is somewhat in tandem with the study conducted by Jagathy et al,10 who reported maximum glucose oxidase activity between pH 5-6. The pH of the culture medium plays a crucial role in modulating enzyme activity by affecting the conformational stability and catalytic efficiency of the enzyme. Glucose oxidase exhibits pH-dependent activity due to its sensitivity to changes in protonation states and interactions with the surrounding environment.
Temperature was seen to play an important role in the activity of glucose oxidase. It was observed that at 4, 30, 35, 40, 45, 50 and 55oC, glucose oxidase activity recorded was 0.726, 0.758, 0.105, 0.735, 0.831, 0.726, 0.772 and 0.753 μmolmin-1 respectively. The data indicates that maximum glucose oxidase activity was recorded at 40°C. Lowest activity exhibited by glucose oxidase was observed at 35°C, suggesting a temperature-dependent response for glucose oxidase activity. This finding does not correlate with the report of Jagathy et al,10 who reported optimum temperature for glucose oxidase activity at 27.5°C. The report of Bhat et al.11 is somewhat close to the findings of this study. He and his team recorded a temperature of 35°Cfor maximum glucose oxidase activity. Enzyme activity is highly sensitive to changes in temperature due to its impact on the enzyme's structural stability, conformational dynamics, and catalytic efficiency. Within a certain temperature range, increasing temperature can enhance molecular motion and substrate binding, leading to increased enzyme activity. At lower temperatures, decreased molecular motion and substrate binding may limit enzyme activity, while at higher temperatures, denaturation and loss of enzyme structure can occur, leading to a decline in activity. Kinetics for glucose oxidase was also investigated in the present study. The kinetic parameters (Vmax and Km) for glucose oxidase produced by the fungus Aspergillus niger was graphically obtained from the plot of 1/V against 1/S (reciprocal of enzyme activity vs reciprocal of substrate concentration) according to the Lineweaver-Burk method. The kinetic values obtained in the glucose oxidase enzyme from Aspergillus niger were Vmax of 5.0 mmol/min and Km of 0.254 mM. The result also showed that as the reciprocal of enzyme activity decreased, the reciprocal of substrate concentration also decreased.
The production of glucose oxidase from Aspergillus niger and its activity are both influenced by multiple factors including incubation days, protein concentration, substrate concentration, temperature, and pH. Optimal conditions for glucose oxidase production appear to be around day 5 of fermentation, with peak activity observed at a temperature of approximately 40°C and a pH range of 5-6. Protein concentration correlates with enzyme activity to some extent and other factors such as substrate concentration and environmental pH also play significant roles. These findings show the necessity for careful optimization of fermentation conditions in industrial processes utilizing glucose oxidase, with a focus on maintaining conditions conducive to maximal enzyme production and activity.16
We want to thank all the researchers who contributed to the success of this research work.
The authors declared that there are no conflicts of interest.

©2025 Mayel, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.