International Journal of
eISSN: 2573-2889


Research Article Volume 7 Issue 1
1Universidad Autónoma de Coahuila, Facultad de Ciencias Químicas, México
2CONAHCYT-Centro de Investigación en Química Aplicada, México
Correspondence: Aide Sáenz Galindo, Facultad de Ciencias Químicas, Blvd. Venustiano Carranza y José Cárdenas Valdés, C.P. 25280, Saltillo, Coahuila, México, Tel +844 223 1929
Received: February 04, 2024 | Published: February 13, 2024
Citation: López WYV, Alvarado CJC, Medica MDD, et al. Evaluation of antibacterial and antioxidant properties of Lavandula officinalis extracts obtained by ultrasound. Int J Mol Biol Open Access. 2024;7(1):15-19. DOI: 10.15406/ijmboa.2024.07.00159
Lavandula officinalis is a Mediterranean plant to which antimicrobial and antioxidant properties are attributed. The objective of the research was to evaluate the chemical composition, antioxidant and antibacterial activity of dried leaves of Lavandula officinalis. For the extraction of bioactive compounds, ultrasound was used, which is an environmentally friendly technique, where a yield percentage of 15.51% was obtained. The chemical composition was evaluated by high-performance liquid chromatography coupled to mass (HPLC-MS), where it was demonstrated that it was possible to extract compounds such as rosmarinic acid, medioresinol, which are the main compounds to which the properties of this plant are attributed. The antioxidant evaluation was carried out by DPPH, ABTS, and FRAP, where inhibition percentages of 60.99 %, 79.86 %, and 0.07 mg Trolox/mL, respectively, were found. The antibacterial activity against Staphylococcus aureus and Escherichia coli was evaluated by the agar diffusion method, finding inhibition halos of 10 mm and 9 mm, respectively. This demonstrates that the bioactive compounds contained in Lavandula officinalis are an alternative to the synthetic compounds currently used in the cosmetic, food, and pharmaceutical industries due to the properties they possess.
Keywords: antioxidants, DPPH, ABTS, FRAP, antibacteriano, Lavandula officinalis
The search for natural products to replace synthetic compounds has acquired great importance because these are widely used in the cosmetic, food, and pharmaceutical industries, and it has been demonstrated in several studies that they are toxic and cause adverse effects.1 The Lavandula officinalis (lavender) plant is presented as an alternative to this problem. Because it contains bioactive compounds that provide antibacterial, antifungal, anti-inflammatory, antioxidant and anti-carcinogenic properties.2 Lavandula officinalis is a plant belonging to the Lamiaceae family, a small shrub with lilac flowers from the Mediterranean.3,4 It’s dried flowers contain a large amount of bioactive compounds, including ursolic acid, rosmarinic acid, carnosol, carnosic acid, linalool, among others.5 These bioactive compounds are also considered inhibitors of pathogens such as Staphylococcus aureus, Escherichia coli, Listeria monocytogenes.5,6
Obtaining these bioactive compounds is affected by various factors depending on the extraction method since conventional techniques are not efficient, as they involve high energy consumption and long extraction times. Therefore, strategies that consume less energy and obtain higher yield percentages are sought, such as non-conventional techniques, including ultrasound.7 Ultrasound is based on the cavitation phenomenon, which consists of the collapse of bubbles, causing the cell wall to break and allowing the release of bioactive compounds.8 The increase in diseases related to oxidative stress, such as cancer, has led the scientific community to focus on the bioactive compounds present in various aromatic plants,9 which is why the objective of this research is to evaluate the antibacterial and antioxidant properties of the bioactive compounds present in the Lavandula officinalis plant, obtained by environmentally friendly methods such as ultrasound.
Extraction of bioactive compounds from dried Lavandula officinalis leaves
The leaves were collected from a plant of approximately 70cm in height; these were left to dry at room temperature for one week. Once dried, they were ground and weighed 100g in an analytical balance (Ohaus), which were placed in a reactor together with 1000 mL of ethanol (Jalmek, 100% purity). The extraction was carried out under ultrasound for 25 minutes and at a temperature of 70°C (using a hybrid Ultrasonic Microwave Cooperative Workstation reactor model XO-SM400).
High-performance liquid chromatography-mass spectrometry (HPLC-MS) of bioactive compounds
Lavandula officinalis extracts were analyzed by HPLC-MS (Varían prostar) with diode array detector (280 nm). 1.8 mL of sample was filtered (0.45μm membranes). Separation of components was performed on a Grace Denali c-18 column at 30°C. Mobile phase A was methanol (wash), B acetonitrile and 3% acetic acid C, flow rate 1mL/min, and injection volume 10μL. Mass analysis was performed using a Varían 500-MS ion trap equipment, electrospray ionization (ESI), capillary voltage 90V, negative mode ([M-H])-m/z) and mass acquisition range 100-2000 m/z.
DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging assay
A calibration curve was performed with TROLOX (sigma-aldrich) with different concentrations ranging from zero to 0.5, in the three antioxidant capacity techniques and 1:10 dilutions of the extracts were prepared. The DPPH reagent was prepared by weighing 1.18 mg of DPPH and placing it in 50mL of ethanol. Subsequently, 190μL of the DPPH reagent and 10μL of the extract dilution were added to a microplate and allowed to stand for 30 min in the dark and finally read in a spectrometer at an absorbance of 517 nm. The percentage inhibition of the DPPH radical and ABTS was calculated using the equation shown below and the calibration curve shown in Figure 1.
% inhibition
ABTS free radical scavenging assay (2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid)
For the development of this technique, two reagents were prepared in equal 1:1 amounts. The first was ABTS, for which 18mg was weighed and placed in 5mL of water. For the second reagent, 3.31mg of potassium persulfate was considered and placed in 5mL of water. Subsequently, both reagents were mixed and left to stand for 16 hours in complete darkness. After the resting time, a dilution of the ABTS reagent was made with ethanol, placing 0.7μL of the ABTS reagent and 20μL of ethanol; with this last solution, the assay was performed. For 190 μL of the ABTS reagent and 10μL of the extract dilution were placed in the microplate, which was left to stand for 30 minutes in the dark, to finally read at an absorbance of 734 nm.
FRAP assay (ferric ion reducing antioxidant capacity)
Three different reagents were prepared in this assay. The first was TPTZ (2,4,6-tri(2-pyridyl)-1,3,5-triazine), for which a dilution of hydrochloric acid (HCl) was prepared by placing 264.54μL of HCl in 100mL of water, then 1mL of the HCl solution was taken, and 3.123mg of TPTZ was added. The second reagent was FeCl3-6H2O, of which 54.058 mg were weighed and placed in 10 mL of water. The third reagent was an acetate buffer with a pH of 3.6. Finally, to prepare the FRAP reagent, a 2:2:20 mixture of the previous reagents was made. In the microplate, 190μL of the FRAP reagent and 10μL of the extract dilution were placed. The microplate was incubated for 15 min in the dark and then read at an absorbance of 593nm. The calibration curve shown in Figure 2 was used to calculate the antioxidant capacity of this technique.
In vitro antibiosis bioassay of Lavandula officinalis extract
Tablets of 0.1g of extract were made. The assay used was agar diffusion, which was performed against a gram-positive bacterium (Staphylococcus aureus) and a gram-negative bacterium (Escherichia coli), which were sown in nutrient broth. With the help of an isopo, Petri dishes were sowed in a fluted manner, and then three tablets of the extract were placed separately. The assay was performed in triplicate, with an absolute ethanol control and an antibiotic control. These were left incubating for 24h at a temperature of 37oC. After the incubation time the presence or absence of an inhibition halo was observed.
When ultrasound was used as the extraction method, a yield percentage of 15.51% was obtained, which is higher than that reported by several authors using conventional extraction techniques. Table 1 shows the different compounds and families that were extracted from the dried leaves of Lavandula officinalis, among which rosmarinic acid, medioresinol and sesaminol stand out. sIn the DPPH test, a loss of coloration is observed, due to the fact that the ethanol solution of this radical has an absorption maximum around 517 nm, while the reduced form has no coloration, so that, in the reaction, as the antioxidant gives up a hydrogen atom to the radical to deactivate it, there is a proportional loss of the violet coloration, as shown in Figure 3&4. While the ABTS technique is based on the neutralization of the cation radical ABTS, which results in a discoloration of green, as shown in Figure 5, this radical is generated prior to the reaction by the addition of potassium persulfate and upon addition of the antioxidant is able to give up an electron or hydrogen atom, as shown in the mechanism depicted in Figure 6. On the other hand, the FRAP method is based on the antioxidant reduction power of the ferric ion, which consists of measuring the reduction of Fe(III) to Fe(II) in acid medium of the iron of the complete TPTZ, obtaining an intense blue complex, as shown in Figure 7.
|
Retention time (min) |
Mass (m/z) |
Compound |
Family |
|
7.42 |
369.3 |
Sesaminol |
Lignans |
|
22.52 |
324.9 |
Ferulyltartaric acid |
Methoxycinnamic acids |
|
23.50 |
259.0 |
Rosmarinic acid |
Hydroxycinnamic acids |
|
28.16 |
387.0 |
Medioresinol |
Lignans |
|
31.79 |
326.9 |
p-cumaroyl tirosina |
Hydroxycinnamic acids |
|
42.29 |
430.9 |
Apigenin 6-c-glucoside |
Flaconoids |
Table 1 Bioactive compounds identified by HPLC-MS of Lavandula officinalis extract
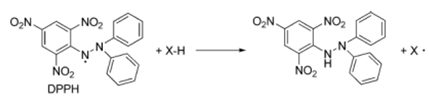
Figure 4 Reaction of the DPPH radical with antioxidant.10

Figure 6 Reaction of ABTS radical with antioxidant.10
These tests showed that the ethanol extracts of Lavandula officinalis contain bioactive compounds that provide antioxidant capacity to the plant through a synergistic effect since it was demonstrated by HPLC-MS that there is a mixture of bioactive compounds. Table 2 shows the results of the antioxidant tests with the different techniques used.
|
Method |
Concentration |
Confidence interval |
|
DPPH |
60.99 % inhibición |
+ 0.1 |
|
ABTS |
79.86 % inhibición |
+ 0.3 |
|
FRAP |
0.07 mg trolox/mL |
+ 0.2 |
Table 2 Antioxidant capacity of Lavandula officinalis extract using DPPH, ABTS and FRAP
It was also demonstrated that the ethanol extract of Lavandula officinalis has antibacterial properties against Staphylococcus aureus and Escherichia coli, using the agar diffusion method. Inhibition halos of 10mm against Staphylococcus aureus and 9mm against Escherichia coli were obtained, while for the antibiotic halos of 18mm were obtained, as shown in Figure 8, demonstrating that the extracts are an excellent alternative for various applications in the food, cosmetic and pharmaceutical industries, due to the properties they have been shown to possess.
In the extraction of bioactive compounds from Lavandula officinalis, a yield percentage of 15.51% was obtained, which is higher when compared to the results obtained in the extraction by conventional methods. Soler,11 et al performed a conventional extraction of dried leaves of Lavandula angustifolia, using maceration, for which they weighed 130g of plant material and placed it in deionized water for 30, 60, 90 and 120 minutes, obtaining yield percentages of 0.26, 0.35, 0.32 and 0.22 %. 11 Perales et al. obtained methanol extracts of Rosmarinus officinalis and Ruta graveolens. The organic material was dried in an oven at 60oC for 48h, to later obtain the methanol extracts using an ultrasound bath at 40Hz with an extraction time of 60 minutes at room temperature and using a ratio of 1:12:5 solid: liquid, where they obtained yield percentages of 0.1% for Rosmarinus officinalis and 3.19 % for Ruta graveolens.12 This demonstrates that by using green technologies such as ultrasound, higher yield percentages are obtained in a shorter extraction time, in addition to the fact that it is an environmentally friendly technology. Three different techniques were used to evaluate the antioxidant capacity of Lavandula officinalis extract, the DPPH (2,2-diphenyl-1-picrylhydroxyl) technique, which is based on the ability of antioxidants to act as free radical scavengers, as well as the ABTS (2,2-azino-bis-3-ethylbenzothiazolin-6-sulfonic acid) technique, while the FRAP method measures the ability of antioxidants to act as reductants.13,14
In DPPH, a color change from purple to yellow was observed when the extract was added to the DDPH reagent, which is indicative of the ability of the bioactive compounds of Lavandula officinalis to inhibit free radicals. Dhouibi et al.15 evaluated the antioxidant capacity of ethanolic extracts of Rosmarinus officinalis obtained by ultrasound, which is a plant belonging to the Lamieaceae family. These were evaluated based on the IC50 values that they determined based on the calibration curve obtained, finding IC50= 0.22 mg/mL, concluding that the extracts of Rosmarinus officinalis present antioxidant properties, due to the fact that they are capable of neutralizing DPPH radicals.15 While Briones et al.16 reported 73.85% inhibition of aqueous extract of Rosmarinus officinalis.16 Rojas et al. evaluated the antioxidant capacity of lavender (Lavandula angustifolia) essential oil by FRAP and ABTS, obtaining results of 88.24μmol Fe2+/g and 101.23μmol trolox/g, respectively.17 The varied chemical composition of the Lavandula officinalis plant, makes it present a high antioxidant activity, especially marked by the presence of phenolic compounds. Rosmarinic acid and medioresinol are among the main compounds to which these antioxidant properties are attributed.18 The agar diffusion test was performed against Staphylococcus aureus and Escherichia coli, where inhibition halos of 10 and 9mm were obtained, respectively. The antibacterial action of Lavandula officinalis is attributed to polyphenols, which produce an enzymatic inhibition of oxidized compounds, by reactions of sulfhydryl groups and by nonspecific interactions with proteins. It has been proven that polyphenols damage the integrity of the bacterial cell membrane, with a greater effect on Gram-positive bacteria, which are more susceptible to the antimicrobial action of bioactive compounds.19–21 The bioactive compounds present in the extracts directly affect the bacterial cell membrane and the cytotoxic activity directly affects the mitotic phase of gram-positive and gram-negative bacteria. Microorganisms such as Escherichia coli, Listeria monocytogenes and Staphylococcus aureus are susceptible to the bioactive compounds of Lavandula officinalis extract, where rosmarinic acid, carnosol, flavonoids, caffeic acid and carnosic acid prevail. 22–24
The bioactive compounds extracted from the dried leaves of Lavandula officinalis showed antioxidant properties by DPPH, ABTS and FRAP, while in the in vitro bioassay, they showed antibacterial properties against Staphylococcus aureus (gram positive) and Escherichia coli (gram negative), with inhibition halos of 10mm and 9mm, respectively. The bioactive compounds work under a synergistic effect because there is a mixture of different compounds, which was observed in the HPLC-MS results, since compounds such as rosmarinic acid, sesaminol and medioresinol were obtained. This demonstrates that the compounds present in Lavandula officinalis present an alternative to avoid the use of synthetic compounds in the pharmaceutical, food and cosmetic industry.
We thank the Universidad Autónoma de Coahuila and the Facultad de Ciencias Químicas, CONAHCYT for the grant awarded and the support provided through the project SEP-CONACYT Ciencias Básica 2017- 2018 CB2017-2018 A1-S-44977.
The authors declare that there are no conflicts of interest.

©2024 López, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.