International Journal of
eISSN: 2573-2889


Research Article Volume 3 Issue 1
1Department of zoology, Mizoram University, India
2Shree Samanvay Institute of Pharmaceutical Education and Research, India
Correspondence: Ganesh Chandra Jagetia, Department of zoology, Mizoram University 10, Maharana Pratap Colony, Sector-13, Hiran Magri, Udaipur-313002, India
Received: November 03, 2017 | Published: January 29, 2018
Citation: Jagetia GC, Rao SK. Detection of DNA strand breaks by fluroscence assisted DNA unwinding (FADU) assay in Hela cells treated with berbrine chloride before exposure to various doses of ?- radition. Int J Mol Biol Open Access. 2018;3(1):14-21. DOI: 10.15406/ijmboa.2018.03.00043
The DNA damage plays an important role in the cell death and DNA double strand breaks in particular have been implicated in cell mortality. Therefore, the ability of berberine chloride to modulate radiation-induced DNA damage in HeLa cells exposed to different doses of γ-rays was studied by fluorescence assisted DNA unwinding assay. The spontaneous frequency of undamaged DNA remained unaltered in HeLa cells treated with 0, 1, 2, 4, 6 or 8µg/ml of BCL at 0, 0.25, 0.5, 1, 2 or 4h post-irradiation. Immediate exposure of HeLa cells to 3 Gy γ-radiation (0h post-irradiation group) caused a drastic rise in the DNA strand breaks as evident by an abrupt reduction in the undamaged double stranded DNA (dsDNA). An elevation in the undamaged ds DNA was observed with time up to 12h post-irradiation in cells exposed to 3Gy irradiation without BCL treatment, indicating repair of radiation-induced DNA damage. However, incubation of HeLa cells with different concentrations of BCL before 3Gy irradiation caused a dose-dependent increase in the DNA strand breaks at all post-irradiation times. The highest DNA strand breaks were observed for 8µg/ml BCL after exposure to 3 Gy. The DNA strand breaks did not show repair up to 12h in cells exposed 3 Gy. The exposure of HeLa cells to 0.25 to 4 Gy γ-radiation resulted in a dose dependent decline in dsDNA at all post-irradiation times and treatment of HeLa cells with 8 µg/ml berberine before irradiation led to a further attrition in the dsDNA. Our study demonstrates that irradiation of HeLa cells resulted in a dose dependent increase in DNA strand breaks and berberine treatment further increased the DNA strand breaks which may be one of the mechanisms of cell death.
Keywords: berberine, hela cells, DNA damage, FADU, irradiation
Ionizing radiations are in frequent clinical use for diagnosis and treatment and the fact is that 50% of the patients visiting Cancer Treatment Centers for cancer therapy receive radiotherapy either alone or in conjunction with chemotherapy.1,2 The radiotherapy and chemotherapy both kills tumor cells by inflicting damage to cellular DNA and it is also known that the underlying cause of cancer is the induction of mutations into DNA by physical or chemical agents.3‒5 The DNA damaging agents, including ionizing radiations and interstrand DNA cross-linking compounds have been used alone or as a combined treatment modality against cancer due to their extreme DNA damaging ability on proliferating cells. The efficacies of radiation therapy and chemotherapy as well as the mutagenic potential of the DNA damaging agents used in these therapies are modulated by the ability of cells to repair the inflicted DNA damage.6 Investigation of the molecular mechanisms of DNA damage repair and maintenance of genome stability and their biological effects are important. One of the most genotoxic DNA lesions that results from treatment with ionizing radiation or DNA cross linking compounds is the DNA double-strand break (DSB). DSBs are caused not only by exogenous sources but also by endogenous sources such as radicals generated during metabolic processes.4‒5 In addition, a predominant source of DSBs in dividing cells is the process of DNA replication itself. Since DNA strand breaks play an important role in mutagenesis and oncogenesis, their dose–effect relationships and their rejoining kinetics are essential indicators that reflect cellular response processes.6‒8 In addition to the primarily induced DNA breaks many other DNA lesions may be transformed into strand breaks by cellular repair processes and can thus easily be measured and quantified.4,5
The Fluorometric analysis of DNA unwinding (FADU) method has been reported to be as sensitive as the alkaline filter elution technique in detecting DNA strand breaks induced by X-rays or after treatment with radical-generating chemicals.9,10 FADU technique has been also useful in the identification of effects of occupational exposure to industrial air pollutants.11 Moreover, FADU assay is 3-4 times more sensitive than unscheduled DNA synthesis in detecting mutagenic effects In vitro by direct acting mutagens or environmental agents in various systems.12,13 The number of free DNA ends may be calculated after alkaline unwinding either from the size-distribution of unwound DNA9 or from the numerical discrimination between ssDNA and dsDNA. In this respect, fluorescent dyes are useful tools. FADU assay is a fast, sensitive and reliable method for the detection of strand breaks in DNA as an index of DNA damaging potential of radiation and/or chemical agents.13
The medicinal plants have a long history of use in the treatment of various ailments including cancer and many plants contain berberine, has formed the part of traditional systems including Ayurvedic and Chinese systems of medicine. Several clinically important medicinal plants, including Arcangelisia Flava , Berberis aquifolium (Oregon grape), Berberis aristata (tree turmeric), Hydrastis canadensis (goldenseal), Coptis chinensis (coptis or golden thread), Radix scutellariae and Tinospora cordifolia (giloy) have shown the presence of isoquinoline alkaloid, berberine. The antibacterial, antimicrobial, antioxidant, antidepressant, and anti inflammatory activities of berberine have been reported earlier.14‒17 It has been also reported to be anti diarrheal, anti arrhythmic, antihypertensive, anti osteoarthritis, chemo sensitizing, hepatoprotective and neuro protective.18‒20 Berberine treatment has been found to protect rats against ischemia-reperfusion injury.21,22 Clinically berberine administration has been reported to reduce glucose, total cholesterol and low-density lipoprotein cholesterol levels in the diabetic patients.23‒25 Clinical trials have indicated that chronic congestive heart failure patients treated with 1.2 to 2g of berberine daily showed an improvement in the left ventricular ejection fraction and ventricular premature complexes.26 In yet another clinical trial berberine has been found to be effective in treating dementia dyslipidemia, hyperlipidemia, ocular Behcet's disease, and non-fatty liver disease.27‒32 The anti angiogenic and anticancer activity of berberine has been reported in several studies.33‒37 Beberine chloride has also been reported to increase the tumor radio sensitivity in irradiated HeLa cells.38 Berberine sulfate has been reported to significantly alleviate tumor yield in the 7,12-dimethylbenz(a) anthracene (DMBA) induced tumors in rats.39 Therefore, the present study was undertaken to study the role of berberine chloride in enhancing the radiation-induced DNA damage in HeLa cells exposed to different doses of γ – radiation by FADU assay.
Minimum Essential Medium (MEM), Fetal calf serum (FCS), Hoechst 33258, try pan blue, sodium dodecyl sulphate (SDS), urea, β-mercaptoethanol, cyclohexane diamine tetra acetate (CDTA), were obtained from Sigma Chemical Company, St. Louis, USA, whereas rest of the chemicals were procured from Ranbaxy Fine Chemicals Limited, Mumbai, India.
Preparation of drugs
Doxorubicin hydrochloride (DOX) or Adrim, a kind gift from Dabur Pharmaceuticals, New Delhi, India and berberine chloride (BCL) were dissolved in sterile double distilled water (DDW) at a concentration of 5mg/ml and diluted in MEM as required. All drug solutions were prepared afresh immediately before use. HeLa S3 cells having a doubling time of 20±2 h were procured from National Centre for Cell Science, Pune, India, and were used throughout the study. The cells were routinely grown in 25cm2 culture flasks (Techno Plastic Products, Trasadingën, Switzerland) containing Eagle's minimum essential medium (MEM) supplemented with 10% fetal calf serum, 1% L-glutamine and 50µg/ml gentamicin sulfate at 37°C in an atmosphere of 5% CO2 in humidified air in a CO2 incubator (Nu Air, Plymouth, USA) with their caps loosened.
A fixed number (5X105) of exponentially growing cells were inoculated into several culture flasks (Techno Plastic Products, Trasadingën, Switzerland) and were allowed to complete two division cycles before the onset of experiments.
Experiment 1: Selection of optimum dose
DDW + Irradiation: HeLa cells were treated with an equivalent amount of DDW before exposure to 3Gy γ-radiation.
DOX + Irradiation: This group of HeLa cells was treated with 2µg/ml DOX, before exposure to 3Gy γ-radiation.
BCL + Irradiation: The cell cultures of this group received 0, 1, 2, 4, 6 or 8µg/ml BCL before exposure to 3Gy of γ-radiation.
DNA strand breaks in both groups were studied at 0, 0.25, 0.5, 1, 2, 4, 6 or 12h post-irradiation.
Experiment 2: Effect of BCL on radiation-induced DNA strand breaks
A separate experiment was performed to study the effect of 8µg/ml BCL on the radiation-induced DNA damage, where groupings and other conditions were essentially similar to that described above except that HeLa cells were treated with 8µg/ml BCL and then exposed to 0, 1, 2, 3 or 4Gy γ-radiation. The DNA damage was evaluated at 0, 0.25, 0.5, 1, 2 or 4h post-irradiation.
Irradiation
After 4 hours of the above treatments, the cells were exposed to either 0 or 3Gy (Experiment No. 1) or 0, 0.5, 1, 2, 3 or 4Gy γ-radiation (Experiment No. 2) from a Tele cobalt therapy source (Theratron Atomic Energy Agency, Ontario, Canada) at a dose rate of 1Gy/min and at a distance (SSD) of 91cm.
Fluorescence assisted DNA unwinding (FADU)
The fluorescence assisted DNA unwinding (FADU) assay detects DNA single strand, double strand breaks and alkali labile sites (DNA breaks). The alteration in radiation-induced DNA breaks by BCL in HeLa cells was assessed using FADU assay in triplicate as described earlier with minor modifications.40 Briefly, the cells were incubated with 1ml of reagent mixture (containing 0.3ml of 6m M myo-inositol, 0.1ml of 2% urea, 0.1ml of 5% SDS, 0.4ml of 5M NaOH and 0.1ml of 2mM CDTA in MilliQ water (Millipore, Billerica, MA, USA) for 1h at 15˚C. The samples were flash frozen for 1min and 0.4ml neutralizing mixture (containing 1M glucose, and 14mM beta mercaptoethanol), was added at room temperature. The samples were immediately sonicated for 5seconds (Sonics Vibra-cell, Newtown, CT, USA) to inhibit re association of complementary strands of DNA and to reduce the molecular weight of DNA. Each cell sample was transferred to a quartz curette and mixed gently with 1ml Hoechst 33258 (5µg/ml) by single inversion and the fluorescence was read at an excitation and emission wavelengths 350nm and 450nm, respectively using a spectrofluorimeter (SFM 25, Kontron Instruments, Neufahrn, Germany) at room temperature.
The percent undamaged double stranded DNA and DNA strand breaks in the treatment groups were calculated as follows:
Undamaged double stranded DNA (%)=(P-B/T-B) x 100
Percent DNA strand breaks DSB(s)=100-percent undamaged double stranded DNA, where
P (Partial de maturation): HeLa cells, reagent mixture but without dye.
B: HeLa cells, DDW/BCL and dye but without reagent mixture.
T: HeLa cells, DDW/BCL, reagent mixture and dye.
The strand scission factor (SSF) is defined as ln Ft/Ft=0 and was calculated as follows: -SSF=log (% dsDNA in sample/% dsDNA in control).
Statistical analysis
The statistical analyses were performed using Graph Pad Prism 6 statistical software (Graph Pad Software, San Diego, CA, USA). The significance among different treatments was determined by one-way ANOVA and Bonferroni’s post-hoc test was applied for multiple comparisons. The experiments were repeated for confirmation of results. The results are the average of five individual experiments. The test of homogeneity was applied to find out variation among each experiment. The data of each experiment did not differ significantly from one another and hence, all the values have been combined and means calculated. A p value of <0.05 was considered statistically significant.
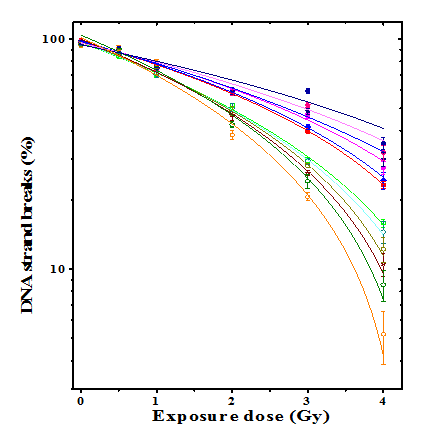
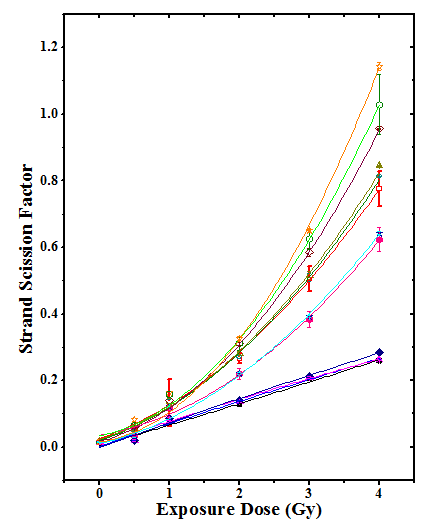
The results are expressed as percent undamaged double stranded DNA±SEM in tables 1-2 and (Figure 1) and strand scission factor ± SEM (SSF±SEM) in (Figure 2).
Experiment 1: Selection of optimum dose
Treatment of HeLa cells with various concentrations of BCL did not alter the baseline frequency of DNA strand breaks as differences between the control and BCL treatment was non-significant except for 6 and 8µg/ml BCL (Table 1). Assessment of DNA damage in HeLa cells immediately after exposure to 3Gy (0h post-irradiation group) caused a drastic reduction in the undamaged double stranded DNA (dsDNA). An elevation in undamaged dsDNA was observed with time up to 12h post-irradiation in cells exposed to 3Gy irradiation without BCL treatment, indicating repair of radiation-induced DNA damage. Treatment of HeLa cells with different concentrations of BCL before 3Gy irradiation caused a dose-dependent reduction in the undamaged dsDNA at all post-irradiation times. The greatest DNA damage was observed for 8µg/ml BCL after exposure to 3Gy. The maximum DNA strand breaks were observed at 4h post-irradiation in HeLa cells treated with various concentrations of BCL before 3Gy irradiation that remained almost unchanged up to 12h post-irradiation after 3Gy irradiation group (Table 1). When the evaluation of dsDNA was carried out at different post-treatment times giving allowance for repair of DNA, it was observed that the frequency of DNA strand breaks increased with the evaluation time for all doses of BCL in conjunction with 3Gy up to 12h post-irradiation (Table 1). Treatment of HeLa cells with different concentrations of BCL before exposure to 3Gy resulted in a BCL concentration dependent elevation in DNA strand breaks as evident by a constant decline in undamaged dsDNA with increasing concentration of BCL (Table 1). Treatment of HeLa cells with 4µg/ml BCL before 3Gy irradiation increased DNA strand breaks by approximately 50% when compared with concurrent non-drug treated 3Gy irradiated cells at 4h post-irradiation time. Therefore, further studies were undertaken using this concentration.
Post-irradiation time (h) |
Undamaged DNA (%)± SEM |
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DDW 10 µl |
DOX 2 µg/ml |
Berberine chloride (µg/ml) |
||||||||||||
1 |
|
2 |
|
4 |
|
6 |
|
8 |
|
|||||
SIR |
IR |
SIR |
IR |
SIR |
IR |
SIR |
IR |
SIR |
IR |
SIR |
IR |
SIR |
IR |
|
0 |
98.49±2.71 |
44.44±1.10 |
97.12±2.63 |
40.72±0.88 |
98.24±2.46 |
41.63±0.96 |
97.58±2.22 |
40.55±0.78 |
96.57±2.04 |
39.75±0.74 |
95.18±2.03 |
33.23±0.71 |
94.89±2.04 |
30.25±1.24 |
0.25 |
98.28±2.36 |
44.6±1.05 |
96.78±2.22 |
39.22±0.69§$ |
98.16±2.18 |
40.34±1.13§$ |
97.52±2.3 |
39.12±0.97§$ |
96.25±2.14 |
39.48±0.88§# |
95.11±2.03 |
33.12±0.74§# |
94.51±2.14 |
29.84±0.88§# |
0.5 |
98.17±2.48 |
45.54±1.16 |
96.47±2.40 |
37.64±0.71§$ |
97.88±2.2 |
39.43±0.86§$ |
97.38±2.24 |
36.52±0.72§$ |
95.86±2.17 |
36.22±0.61§# |
95.02±2.14 |
31.05±0.84§# |
94.24±2.34 |
27.29±0.84§# |
1 |
98.14±2.51 |
46.52±1.43 |
96.11±2.53 |
35±0.72§$ |
97.56±2.38 |
35.34±1.12§$ |
97.01±2.17 |
33.45±0.89§$ |
95.66±2.12 |
32.68±0.66§$ |
94.84±2.03 |
26.02±0.89*# |
94.03±2.05 |
23.84±1.02*# |
2 |
98.01±2.37 |
48.55±1.22 |
95.76±2.24 |
33.39±0.5§$ |
97.27±2.15 |
34.28±1.06§$ |
96.34±2.07 |
33.36±0.94§$ |
95.24±1.96 |
32.06±0.7§$ |
94.41±1.88 |
25.85±0.85*# |
93.82±1.87 |
20.55±0.65*# |
4 |
97.87±2.45 |
49.35±1.33 |
95.33±2.12 |
30.75±0.39§$ |
97.04±2.11 |
33.78±1.12§$ |
96.22±1.92 |
32.74±0.98§$ |
94.75±1.89 |
31.75±0.88*# |
93.76±0.98 |
25.31±0.67*# |
93.48±2.05 |
19.35±0.25*# |
6 |
97.81±2.34 |
48.76±1.4 |
95.16±2.42 |
30.7±0.43§$ |
96.77±1.99 |
33.44±1.28§$ |
96.01±1.98 |
32.21±1.1§$ |
94.58±1.83 |
29.64±0.83*# |
93.69±2.05 |
24.22±0.88*# |
93.10±2.36 |
15.52±1.02*# |
12 |
97.81±2.5 |
48.25±1.52 |
95.83±2.13 |
30.66±0.44§$ |
96.26±2.04 |
33.38±1.31§$ |
95.51±1.88 |
32.36±1.23§$ |
94.04±1.89 |
28.16±0.84*# |
93.41±2.15 |
23.84±1.24*# |
92.78±1.87 |
12.36±0.95*# |
Table 1 Effect of various concentrations of berberine chloride on the radiation-induced DNA strand breaks in HeLa cells exposed to 3 Gy- radiation
*=p<0.0001; =p<0.001(When IR groups were compared with DDW+IR); #=p<0.002; $=p<0.05(When IR groups are compared with SIR); No symbols=Non-significant. SIR, Sham-irradiation (0Gy); IR, Irradiation; DOX, doxorubicin hydrochloride; BCL, berberine; DDW, double distilled water
Experiment 2: Effect of BCL on radiation-induced DNA strand breaks
Spontaneous DNA strand breaks in HeLa cells were approximately 2.5%. Irradiation of HeLa cells to various doses of γ-radiation resulted in a dose-dependent increase in DNA strand breaks at all post-irradiation times and the highest strand breaks were estimated at 0h post-irradiation for all exposure doses (Figure 1). A time dependent repair in DNA strand breaks was observed in the DDW + irradiation group, and a maximum repair was discernible at 4h post-irradiation for 0.5Gy irradiation, where only 10% of DNA was found to be damaged (Table 2). The repair of DNA strand breaks was minimal for 4Gy even up to 4h post-irradiation as 65% of the DNA still remained damaged (Table 2). Treatment of HeLa cells with 8µg/ml of BCL before irradiation to various doses of γ-rays resulted in a significant elevation in the DNA strand breaks at all post-irradiation times. The cells exposed to 0.5Gy and 1Gy after BCL treatment showed repair of damaged DNA with time and a maximum repair of DNA was observed at 4h post-irradiation (Table 2). However, treatment of HeLa cells with 8µg/ml BCL before exposure to 2-4Gy resulted in a continuous elevation in damaged DNA up to 4 h post-irradiation where 95% of the DNA remained damaged after 4Gy exposure in BCL + irradiation group (Table 2).
|
Undamaged DNA (%)± SEM |
|
|
|
|
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Post-irradiation time (h) |
|
|
|
|
|
|
|
|
|
|
|
Exposure dose (Gy) |
0 |
|
0.25 |
|
0.5 |
|
1 |
|
2 |
|
4 |
|
DDW+IR |
BCL+IR |
DDW+IR |
BCL+IR |
DDW+IR |
BCL+IR |
DDW+IR |
BCL+IR |
DDW+IR |
BCL+IR |
DDW+IR |
BCL+IR |
|
0 |
97.41±2.71 |
95.42±2.04 |
97.58±2.36 |
95.45±2.14 |
97.12±2.28 |
94.86±2.07 |
97.44±2.01 |
94.35±2.20 |
97.05±2.24 |
94.20±2.01@ |
96.73±2.08 |
94.09±2.11@ |
0.5 |
90.12±2.04 |
84.55±1.86 |
90.21±1.85 |
85.32±1.56@ |
90.43±1.68 |
87.65±2.04@ |
90.52±1.67 |
89.26±2.05@ |
90.31±2.14 |
90.16±1.17# |
90.23±2.31 |
91.33±2.33# |
1 |
74.54±1.82 |
69.72±1.38@ |
74.73±1.87 |
70.22±1.88# |
74.94±1.88 |
72.34±2.12# |
75.08±1.66 |
74.65±1.87# |
75.24±2.05 |
77.37±1.11# |
75.48±1.95 |
79.23±2.14a |
2 |
58.73±1.45 |
51.34±1.1@ |
58.75±2.14 |
50.25±2.03# |
58.81±1.66 |
48.54±2.15# |
58.96±2.14 |
46.62±2.14# |
59.11±2.36 |
42.51±1.16a |
59.42±1.88 |
38.33±1.57a |
3 |
39.87±1.10 |
29.81±0.74@ |
41.58±1.05 |
29.52±0.88# |
46.38±1.16 |
28.19±0.61# |
48.51±1.43 |
26.08±0.66a |
51.61±1.12 |
24.11±1.70a |
59.44±1.33 |
20.66±0.88a |
4 |
23.25±0.84 |
15.87±0.64@ |
24.31±2.02 |
14.54±1.58# |
27.55±1.84 |
12.23±1.56# |
29.94±2.14 |
10.54±1.21a |
32.14±2.34 |
8.56±1.32a |
35.22±2.01 |
5.21±1.36a |
Table 2 Alteration in the radiation-induced DNA strand breaks in HeLa cells treated with 8µg/ml berberine chloride before exposure to different doses of - radiation
a=p<0.0001; #=p<0.001; @=p<0.05(Comparison between DDW and BCl groups); No symbols=Non-significant BCL, berberine; DDW, double distilled water; IR, Irradiation
Effect of BCL on strand scission factor (SSF)
DNA damage has also been expressed as strand scission factor (SSF) for unwound DNA after alkaline treatment. Irradiation of HeLa cells to 0, 0.5, 1, 2, 3 or 4Gy, caused a significant and dose dependent elevation in SSF at all post-irradiation times (Table 3). The pattern of increase of SSF was identical in BCL+irradiation group after exposure to different doses of γ-irradiation except that SSF was significantly higher in this group when compared with the DDW+irradiation group at all post-irradiation times (Figure 2). The strand scission factor however, was higher for BCL treatment at all irradiation doses when compared with DDW+irradiation group (Table 3). The strand scission factor in BCL+irradiation group was approximately 1.6 folds greater than DDW + irradiation group for 2h post-irradiation for 0.5 to 3Gy gamma radiation, whereas, it was 4 folds greater for 4Gy irradiation (Table 3).
Exposure dose (Gy) |
Strand scission factor (SSF)±SEM |
|
|
|
|
|
|
|
|
|
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Post-irradiation time (h) |
|
|
|
|
|
|
|
|
|
|
||
|
0 |
|
0.25 |
|
0.5 |
|
1 |
|
2 |
|
4 |
|
|
DDW+IR |
BCL+IR |
DDW+IR |
BCL+IR |
DDW+IR |
BCL+IR |
DDW+IR |
BCL+IR |
DDW+IR |
BCL+IR |
DDW+IR |
BCL+IR |
0 |
0.01522±0.002 |
0.01574±0.006 |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
0.5 |
0.01799±0.001 |
0.04896±0.003 |
0.0188±0.002 |
0.05132±0.003 |
0.02014±0.002 |
0.0543±0.003 |
0.02052±0.007 |
0.0593±0.003 |
0.03498±0.002 |
0.0656±0.003¨ |
0.03610±0.007 |
0.0821±0.00¨ |
1 |
0.0702±0.0056 |
0.132±0.071¬ |
0.0824±0.001 |
0.134±0.008¬ |
0.08541±0.005 |
0.138±0.008¬ |
0.08654±0.008 |
0.1411±0.00¬ |
0.1174±0.0078 |
0.1584±0.01¨ |
0.1210±0.001 |
0.1635±0.00¨ |
2 |
0.1279±0.0087 |
0.265±0.016¬ |
0.1354±0.004 |
0.271±0.004¬ |
0.1367±0.002 |
0.282±0.004¬ |
0.1417±0.004 |
0.3108±0.004¨ |
0.2209±0.0164 |
0.3118±0.021a |
0.2213±0.002 |
0.3254±0.006a |
3 |
0.2056±0.012 |
0.505±0.038¬ |
0.2074±0.005 |
0.510±0.012¬ |
0.2081±0.004 |
0.5158±0.012¨ |
0.2121±0.004 |
0.5854±0.012a |
0.3836±0.023 |
0.625±0.034a |
0.3916±0.003 |
0.6512±0.001a |
4 |
0.2578±0.015 |
0.775±0.052¬ |
0.2615±0.002 |
0.812±0.002¨ |
0.2643±0.005 |
0.8454±0.002¨ |
0.2844±0.005 |
0.9561±0.002a |
0.6234±0.035 |
1.028±0.088a |
0.6422±0.005 |
1.142± 0.012a |
Table 3 Alteration in the strand scission factors (SSF) in HeLa cells treated with 8µg/ml berberine chloride before exposure o different doses of γ-radiation
a=p<0.001; •=p<0.004; =p<0.05 (Comparison between DDW and BCL groups); No symbols=Non-significant. BCL, berberine; DDW, double distilled water; IR, Irradiation
When ionizing radiation passes through matter, atoms are randomly ionized and excited. Because excited and ionized atoms initiate numerous chemical reactions, the passage of ionizing radiation through a cell produces abnormal alterations in the chemical properties of DNA, i.e., DNA damage.41,42 Cells are exposed to many agents that result in damage to its DNA. These agents include ultraviolet radiation (UV-C), ionizing radiation (γ- and X-rays), reactive oxygen radicals, environmental chemicals, and therapeutic chemicals. The faithful repair of DNA double-strand breaks (DSBs) is probably one of the most critical tasks for a cell in order to maintain its genomic integrity since these lesions may lead to chromosome breaks or rearrangements, mutations and subsequently the cell death.5,43,44 DNA damage is most likely responsible for initiating the harmful biological effects of ionizing radiation, and it is reasonable to assume that the repair of DNA damage has a significant influence on the expression of DNA damage. Ionizing radiation initially creates various types of DNA damage and competition between repair processes and processes converting potentially repairable forms of DNA damage into irreversible forms of damage affect the probability of cell survival after irradiation.45‒47 Most naturally occurring DSB(s), particularly the medically relevant are produced by ionizing radiation and that of some chemotherapeutic agents result from oxidative processes. The predominant repair pathway for repairing DNA DSB induced by ionizing radiation is non-homologous end joining (NHEJ).46,48 The optimal repair conditions vary depending upon the chemical structure of the DSB end being rejoined.49 In addition to DSB(s) induced by γ-radiation, the nucleotides proximal to the DSB site may have also become damaged and the extent of such damage limits the repair by NHEJ pathway.50,51
The treatment of HeLa cells with 8µg/ml BCL before exposure to 2-4Gy resulted in a continuous elevation in damaged DNA up to 4h post-irradiation and 95% of the DNA was damaged after 4Gy exposure in BCL + irradiation group as majority of the DNA damage could not be repaired. This may be the reason for enhanced radiosensitivity of HeLa cells in our earlier study.38The sensitivity of FADU method is comparable to alkaline elution method of measurement of DNA strand breaks.52 Fluorometric analysis of DNA unwinding (FADU assay), first reported by Birnboim and Jevcak to detect X-ray–induced DNA damage.40 It is a fast and reliable technique to detect single-strand DNA breaks as an index of DNA damage induced by genotoxic agents. The fluorescent dye selectively binds to the double stranded DNA in the presence of single stranded DNA whose short duplex regions are destabilized by alkali. DNA lesions other than In situ strand breaks will not affect the rate of strand separation or be labile in alkali.53 Our experimental data indicate that the FADU method is highly sensitive to analyze the DNA strand breaks induced by γ-radiation and the same has been reported by other authors, where they have been able to detect the damage produced by 1-10cGy to 3Gy of ionizing radiation.10,54‒57 BCL has increased the strand breaks induced by γ-rays up to 4 h in the present study.
The exact mechanism of induction of DNA damage by BCL is not known. The increased DNA damage may not be ascribed to a single mechanism and several putative mechanisms may be responsible for the increase in the induction of radiation-induced DNA damage by BCL. Ionizing radiation interacts with cellular genome by induction of ×OH free radicals and the presence of BCL might have further increased the free radicals as it is known to induce free radicals.58‒60 BCL has been found to be effective in inducing DNA damage by increasing the strand breaks without allowing repair of DNA damage by any of the repair pathways including NHEJ since the DNA damage constantly increased up to 4h in the present study. The action of topoisomerase II on DNA leads to its relaxation before transcription and its replication of DNA by cutting one strand of DNA duplex and passing a second duplex through this transient cleavage, the “Cleavable complex”.61‒63 The presence of BCL may have stabilized the cleavable complex and thus increased the DNA double-strand breaks in the present study. Berberine triggers inter nucleosomal DNA fragmentation and inhibit topoisomerase I and II enzymes In vitro.64,65 The primary damage consists of the enzyme covalently bound to DNA. Drug-stabilized covalent complexes are reversible when the drug is removed, but they can be converted into irreversible damage by collision with enzymes tracking along DNA, such as DNA polymerases. Many topoisomerase-targeting drugs are superb probes for DNA repair functions, since they have been shown to be highly specific for their targets. At molecular level suppression of PARP and NF-κB by berberine may have played an important role in suppressing the repair of DNA strand breaks as berberine has been reported to suppress PARP and NF-κB activation and inhibit homologous recombination repair.66 It is clear from our study that BCL has enhanced the geotoxic effects of radiation by increasing DNA damage in the form of strand breaks.
Treatment of HeLa cells with BCL before exposure to different doses of γ–radiation increased radiation-induced DNA damage. The increase in the radiation-induced DNA strand breaks by BCL may be due to increased oxidative stress in the form of free radicals, inhibition of topoisomerase I and II activities. At molecular level BCL may have down modulated the transcription of PARP and NF-κB and suppressed both the NHEJ and homologous recombination repair, which would have increased the DNA strand breaks after combination treatment.
The authors wish to thank Prof. M.S. Vidyasagar and Dr. J. G. R. Solomon, Department of Radiotherapy and Oncology, Kasturba Medical College, Manipal, India, for providing the irradiation facilities and for help in radiation dosimetry, respectively. The financial Assistance from Indian Council of Medical Research and Council of Scientific and Industrial Research, Government of India, New Delhi to carry out the above study is gratefully acknowledged.
Author declares that there is no conflict of interest.

©2018 Jagetia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.