International Journal of
eISSN: 2573-2889


Research Article Volume 5 Issue 4
Department of periodontics, College of Dental Sciences, Rajiv Gandhi University, India
Correspondence: Dr. Vandana KL, MDS, Senior Professor, Department of Periodontics, College of dental Sciences, Rajiv Gandhi University, Karnataka, India, Tel (08192) 231285
Received: September 02, 2020 | Published: October 22, 2020
Citation: Kour H, Vandana KL. Assessment of dentinal hypersensitivity in fluorosed and non fluorosed subjects–a clinical study. Int J Mol Biol Open Access. 2020;5(4):135-143. DOI: 10.15406/ijmboa.2020.05.00140
Background & objectives: Dentin Hypersensitivity(DH) is an exaggerated response to a sensory stimulus that usually causes no response in a normal, healthy tooth which hinders the ability to control dental plaque and compromises oral health. Dental fluorosis is an unfortunate side-effect of fluoride's caries-protective benefits, which brings about mineralisation changes in a dentinal structure leading to dentin hypersensitivity.
Methodology: 404 healthy subjects with and without fluorosis were examined for Dentinal hypersensitivity using tactile and air sensitivity methods. The subjects rated the pain associated with Dentinal hypersensitivity on categorical Verbal rating scale (VRS). Clinical parameters including Turesky modification of Quigley-Hein plaque index (TMQH), gingival recession, labioversion and wasting diseases (attrition, abrasion, erosion,abfraction) were recorded. The tooth brushing habits used by the subjects were also considered.
Results: Results showed that fluorosed subjects were suffering from DH significantly greater than non-fluorosed subjects, giving a prevalence of 22.7% and 11.1% in fluorosed and nonfluorosed subjects, respectively. (p<0.001) The severity of DH increased proportionally with increase in severity of fluorosis. Significantly increased plaque scores were observed in fluorosed teeth. (p<0.05) Gingival recession, labioversion as well as wasting diseases were significantly associated with DH. (p<0.05) Females had significant esthetic concern, psychological impact of dental fluorosis and had a better attitude and desire to seek dental treatment compared to males. Overall, there was limited knowledge about the etiology and
prevention of dental fluorosis in the study subjects.
Interpretation and conclusion: There is a greater prevalence of dentinal hypersensitivity in subjects with dental fluorosis which could be due to fluoride induced enamel and dentinal structural changes leading to rough surface morphology and greater plaque accumulation. There is a need to educate the population regarding the dental effects of fluoride and its management.
Keywords: dentin sensitivity, fluorosis, dental plaque, pain, prevalence, gingival recession, tooth brushing
Dentinal hypersensitivity is an exaggerated response to a sensory stimulus that usually causes no response in a normal healthy tooth. Dental Fluorosis is one of the common complaints of subjects hailing from high water fluoride areas of Davangere district, Karnataka, India. Dental fluorosis is an unfortunate side-effect of fluoride's caries-protective benefits.1 Dental fluorosis (DF) is a tooth malformation believed to be caused by chronic ingestion of high levels of fluoride (F) during tooth development,2 especially from drinking water containing high concentrations of fluoride. Fluorosed enamel is structurally weak and prone for increased enamel attrition.3 It was also shown that the high fluoride content of the tooth decreased the mineralization rate in the tooth’s structure resulting in wider dentinal tubules.4 This knowledge can be broadened to include teeth affected by fluorosis as a cause of dentinal hypersensitivity.
However medline search using key words ‘fluorosis’ and ‘dentinal hypersensitivity’ revealed two studies. One of these studies from endemic fluorosis area reported that fluorosed subjects suffer more tooth sensitivity while other study reported low levels of dentinal hypersensitivity influorosed belt. Other factors which are implicated in the etiology of dentinal hypersensitivity like wasting diseases, labioversion have not been addressed in this study, which could have resulted in a bias while diagnosing a tooth hypersensitivity.5
The two existing studies on fluorosis and dentinal hypersensitivity are contradicting and lack extensive data on this topic that led us to conduct this study in geographically endemic fluorosed area by improvising the short comings of the above two studies. Therefore, the current study was conducted to ascertain if dental fluorosis can serve as an etiology for dentinal hypersensitivity as well to determine the other factors that would contribute to sensitivity of teeth with fluorosis.
The subjects for the present cross sectional case control study were selected from the Department of Periodontics, College of Dental Sciences, Davangere District, Karnataka, India. Davangere district is one of the endemic fluorosis areas of India and therefore, has geographical advantage for studying the association between dental fluorosis and dentinal hypersensitivity. A total of 404 subjects with and without dental fluorosis with no attachment loss, were selected which included both sexes, satisfying the following criteria. The ethical committee approved the study and informed consent was obtained. Subjects with known systemic disorders causing or predisposing to development of dentinal hypersensitivity (e.g. chronic acid regurgitation), history of any disease requiring drugs such as analgesics, tranquilizers or mood altering medications, who had undergone periodontal therapy prior to 6 months of examination, who had used desensitizing toothpaste or mouth rinse prior to 6 weeks of examination, teeth having cracks or fractures in the enamel, caries, restoration of any type or endodontic problems, non-carious cervical lesions in the enamel, abutment teeth for dentures or orthodontic appliances and third molars were excluded from the study.
Subjects who participated in the study were divided into Fluorosed (FL) and non-fluorosed (NFL) groups. The Jackson’s Fluorosis index was used to assess dental fluorosis status.6 The subjects who replied positively for the symptoms of dentinal hypersensitivity were recruited in the study and all their teeth were clinically examined. The subjects who had at least one tooth that responded positively to cold air or tactile stimulus were identified as having dentinal hypersensitivity were recruited.
The demographic data such as age, sex and tooth brushing habits, drinking water source of the selected subjects were recorded. Following which the clinical parameters recorded were Turesky modification of Quigley-Hein plaque index (TMQH) 1970,7 gingival recession,8 presence of labioversion and wasting diseases (attrition, abrasion, erosion, abfraction).
A total of 294 teeth were excluded from the study because they had one or more of the exclusion criteria; 3859 teeth were evaluated for sensitivity. For the dentinal hypersensitivity assessment, the subjects who responded positively were asked to quantify their sensitivity levels by making a mark on the verbal rating scale (VRS). The VRS is a 5-point scale consisting of a list of phrases that describe increasing levels of pain intensity. Respondents select the single phrase that best characterizes their pain intensity.9,10 The sensitivity levels of the teeth were evaluated using tactile and cold air stimuli. Tactile sensitivity was assessed using a Shepherd’s hook explorer, which was applied perpendicular to the cervical third of each tooth and the tip of the explorer was used to scratch the surface in horizontal direction.11,12
Ten minutes after the tactile stimulus, the patient’s response to cold air stimulus was assessed using a blast of cold air from dental unit triple syringe applied approximately 2 mm from and perpendicular to the tooth surface which was isolated from neighbouring teeth with cotton rolls, for 1 second.12,13 Immediately after each application, the patients were asked to qualify their sensitivity using the VRS for each tooth. The phrases selected by the subjects who responded positively were recorded as tactile stimulus VRS and cold air stimulus VRS, respectively, for each tooth.
Statistical analysis
Descriptive statistics including standard deviations and frequency distributions were constructed using a statistical package software program (SPSS 13.0, SPSS Inc. IL, USA). The Chi-square test [X2] was used to compare the presence of tooth sensitivity, gender distribution and age distribution of the groups. Paired t-test was used to for intergroup comparison of plaque levels and z test of proportions was used to compare categorical data between FL and NFL groups.
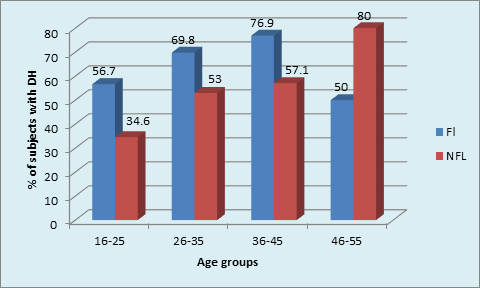
This study was conducted from October, 2012 to June, 2014 during which a total of 9890 subjects visited to the Department of Periodontics. Out of 404 subjects selected 51.2 (207) were males and 48.7 (197) were females. The results of the following study are expressed in Tables 1–3 and Graphs 1A,1B,2A,2B,3A,3B,4,5,6,7,8,9 and 10.In the current study, the different age groups were matched between FL and NFL groups. No correlation was found between age and prevalence of dental fluorosis indicating that age is not a confounding factor for fluorosis. (Table 1, Graph 1A)
Age |
FL n (%) |
NFL n (%) |
Total n (%) |
x2 |
p value |
16-25 |
133 (51.2) |
127 (48.8) |
260 (100) |
4.88 |
0.181, NS |
26-35 |
44 (40.0) |
66 (60.0) |
110 (100) |
||
36-45 |
13 (48.1) |
14 (51.9) |
27 (100) |
||
46-55 |
2 (26.6) |
5 (71.4) |
7 (100) |
||
Total |
192 (47.5) |
212 (52.5) |
404 (100) |
Table 1 Distribution of sample based on age in FL and NFL subjects
¶ Chi-square test, NS: Non-significant
|
FL |
|
NFL |
|
|
|
||
Age |
DH present n (%) |
DH absent (%) |
Total n (%) |
DH present n (%) |
DH absent n (%) |
Total n (%) |
z§ |
p§ value |
16-25 |
76 (56.7) |
58 (43.3) |
134 (100) |
44 (34.6) |
83 (65.4) |
127 (100) |
2.55 |
0.01, S |
26-35 |
30 (69.8) |
13 (30.2) |
43 (100) |
35 (53.0) |
31 (47.0) |
66 (100) |
1.74 |
0.008, NS |
36-45 |
10 (76.9) |
3 (23.1) |
13 (100) |
8 (57.1) |
6 (42.9) |
141(100) |
1.08 |
0.27, NS |
46-55 |
1 (50.0) |
1 (50.0) |
2 (100) |
4 (80.0) |
1 (20.0) |
5 (100) |
0.79 |
0.42, NS |
Total |
117 (69.9) |
75 (39.1) |
192 (100) |
91 (42.9) |
121 (67.1) |
212 (100) |
6.05 |
0.10, NS |
¶ x2 |
3.9 |
10.26 |
- |
- |
||||
¶ p |
0.25, NS |
|
|
0.01, S |
|
|
|
- |
Table 2 Distribution of DH within age groups in FL and NFL subjects
¶ Chi-square test, NS: Non-significant; S: Significant; § z test for proportions
Fluorosis index |
Absent |
A |
B |
C |
D |
E |
F |
Total |
Number |
1928 |
283 |
699 |
303 |
249 |
82 |
315 |
3859 |
% |
50 |
7.3 |
18.1 |
7.9 |
6.5 |
2.1 |
8.2 |
100 |
Table 3 Number of in NFL group and FL group according to Jackson’s fluorosis index
Graph 1B Distribution of DH within age groups in FL and NFL subjects:16-25 years: (p value= 0.01, Significant).
Graph 2B Distribution of sample based on gender in FL and NFL subjects: Males and females in FL group (p <0.001, Highly Significant).
Graph 3A VRS characteristics using TS and AS methods in FL and NFL teeth: DH and fluorosis in AS method (p < 0.001, Highly Significant).
Graph 3B Prevalence of DH in FL and NFL teeth using TS & AS methods: fluorosis and DH (p < 0.001, Highly Significant).
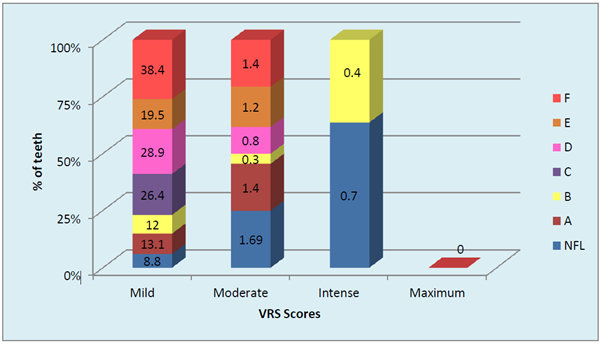
Graph 4 Distribution of severity of dentinal hypersensitivity in FL and NFL teeth Severity of fluorosis and severity of dentinal hypersensitivity in terms of VRS scores (p <0.001, Highly Significant).
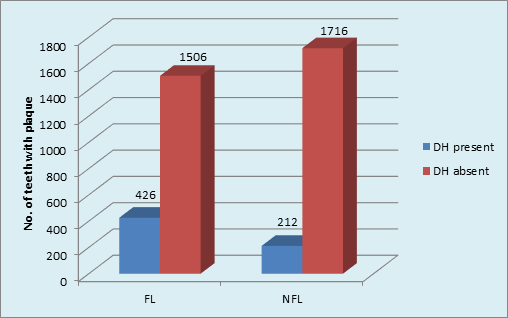
Graph 5 Distribution of sample based on plaque and DH in FL and NFL groups: DH in FL group(p <0.001, HS); DH in NFL group (p <0.001,HS).
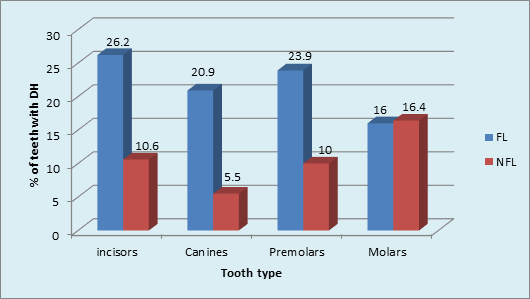
Graph 6 Tooth type most commonly affected by dentinal hypersensitivity in FL and NFL groups: Incisors, canines, premolars (p value <0.001, Highly Significant).
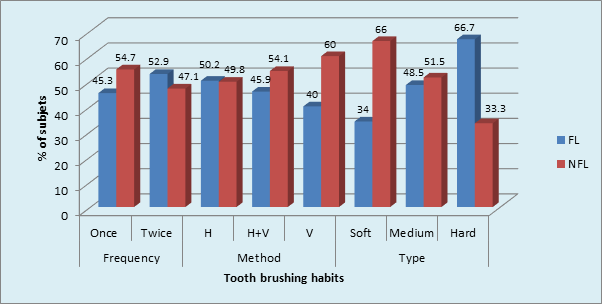
Graph 7 Prevalence of Tooth brushing habits in sample population: Medium toothbrush in FL and NFL (p = 0.02,Significant) .
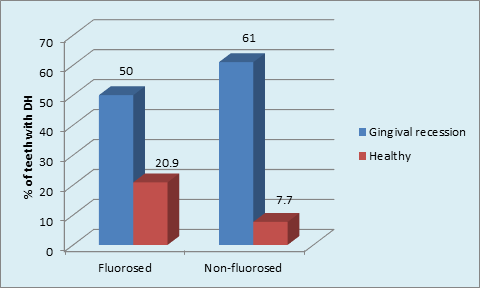
Graph 8 Distribution of the sample based on presence GR and DH in FL and NFL groups, Gingival Recession having DH in NFL group (p <0.001, highly significant); DH and GR in FL teeth ( p = 0.001, Significant) & NFL teeth ( p < 0.001, highly significant).
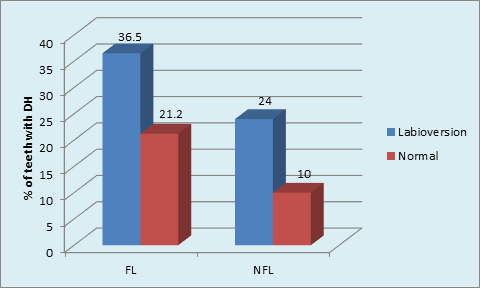
Graph 9 Distribution of sample based on Labioversion and DH in FL and NFL groups: labioversion and DH in FL group (p = 0.001, Significant); labioversion and DH in NFL group(P<0.001, Highly Significant).
The presence of DH amongst different age cohorts was found to be similar in FL group, unlike the significantly higher (80%) dentinal hypersensitivity(DH) in age cohorts of (46-55 years)NFL group. The presence of DH was not significant between FL and NFL groups in any age cohorts except in 16-25 years where it was significantly higher in FL (56.7%)subjects as compared to 34.5% of subjects in NFL group. (p = 0.01, S) (Table 2, Graph 1B)
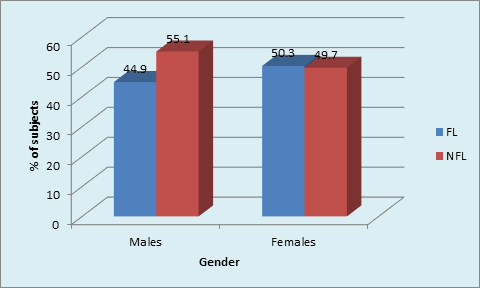
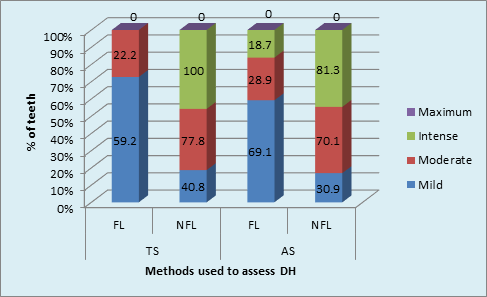
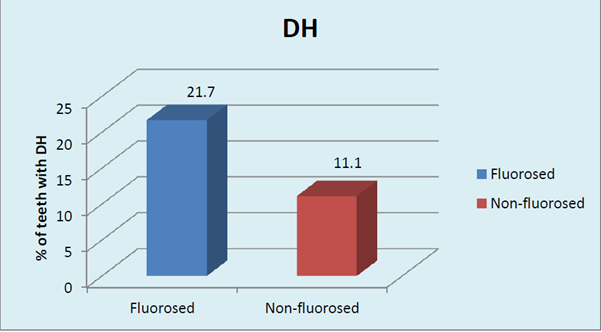
The gender based distribution of sample was not significant between FL and NFL groups. Sex matching was observed. No significant correlation was found between gender and prevalence of fluorosis. (Graph 2A). DH was significantly higher in both males and females in FL group compared to NFL group (p <0.001, HS). (Graph 2B). Mild to moderate pain due to DH was observed in both F & NFL teeth which were significantly higher in FL teeth using both AS & TS methods. Intense pain was found in one NFL tooth using TS method and 18.7% & 81.3% in FL & NFL teeth using AS method. (Graph 3A) Irrespective of the method used, 21.7% teeth had DH in the FL group compared to 11.1% in the NFL group. (Graph 3B). The order of number of teeth with different grades of Jackson’s fluorosis index was B>F>C >A >D>E. (Table 3) Chi-square analysis (x2 =2.87; p <0.001, HS) showed a highly significant correlation between severity of fluorosis and severity of dentinal hypersensitivity in terms of VRS scores. (Graph 4)
Intergroup comparison showed that a higher percentage (22%) of DH was found in FL than in NFL teeth (11%) [p <0.001, HS]. The role of plaque in inducing DH was obvious in FL teeth. (Graph 5) The type of NFL teeth affected maximum with DH was Molars (16.4%) followed by minimum in Canines 5.5%. The order of tooth type affected with DH in NFL group was: Molars>incisors>premolars>canine. Chi-square analysis (x2 = 22.7, p <0.001, HS) showed significant results. (Graph 6).
Maximum number of subjects used medium tooth brush in FL (48.5%) and NFL (51.5%) groups. (p = 0.02, S) (Graph 7) The maximum type of GR was shallow narrow 34 (52.3%) in FL group and shallow wide 77 (77.0%) in NFL group. The gingival recession and dentinal hypersensitivity irrespective of the fluorosis status (Graph 8) showed significantly higher percentage (36.5%) of labioversion teeth with DH in NFL group as compared to FL group on inter group comparison.(p = 0.04, S) (Graph 9).
Intergroup comparison using z test for proportions showed significantly higher number of( 394 ,22.7%) teeth without wasting diseases having DH in FL group compared to NFL group 119 (7.6%). (p = 0.04) Intergroup comparison for attrition, abrasion, erosion, abfraction was not significant between FL and NFL teeth. (p> 0.05) (Graph 10).
Dentinal hypersensitivity can be a potential threat to individual’s oral health because such pain may interfere with the maintenance of good oral hygiene. However, it still remains a poorly understood area and consequently there appears to be no permanent treatment for this clinical condition. The dentinal tubular, patency, diameter plays an important role in causation of dentinal hypersensitivity. Microscopic examination reveals that patent dentinal tubules are more numerous and wider in hypersensitive dentine than in non-sensitive dentine. It has been reported in literature that hypomineralized teeth are extremely sensitive to cold and sweet stimuli as well as tooth brushing. Moreover, fluoride induced structural changes in dentine have been investigated which demonstrated fluorides influence on crystal size leading to wider dentinal tubules in fluorosed teeth.14,15 This was the first study to be conducted on the prevalence and clinical features of DH in subjects with dental fluorosis in Indian population. The results of the study are discussed as follows: The presence of Dentinal hypersensitivity (DH) amongst different age cohorts was found to be similar in FL group unlike the significantly higher DH in age cohorts of NFL group.
Various authors have reported similar age group of 40-49 years with higher DH.16–20 However, other age groups with higher DH reported are 50-59 years,21 20‑39 years,22 20 and 40 years, 20 and 25 years,23 25 and 29 years.24 The different age distribution of dentine hypersensitivity prevalence for different studies could arise from the age compositions of the study populations. The increased cervical dentine exposure with age peaked between 40-50 years followed by a decline with age.13 the age related changes in pulp-dentine complex such as dentinal sclerosis and laying down of secondary and tertiary dentine may be responsible for the drop in DH.19 The significantly higher DH in 16-25 years in FL group requires to be further evaluated. This could probably due to post-eruptive enamel breakdown that the fluorotic teeth suffer because of structural changes like increased porosity, higher protein levels, and lower amounts of minerals and, in severe cases, the formation of a pitted surface leading to exposed dentine.25 The situation is complicated as this exposed dentine has been proved to have wider dentinal tubules predisposing them to DH.14,15 As age advances, probably age related pulp-dentine changes such as formation of secondary and tertiary dentine formation may reduce the DH in fluorosed subjects. For the first time, DH and different age cohorts are studied in flurorosed and non-fluorosed subjects. There is a need to increase the sample size in each cohort to confirm the results of this study.
The distribution of males and females between fluorosed and non-fluorosed groups were similar. Gender is a confounder while measuring DH as it has been found that women report pain more frequently, and have a lower threshold for pain than men.26 The gender matching which was done in this study minimizes the error of gender bias. Al-Wahadmi & Linden while studying dentinal hypersensitivity in Jordanian dental attenders considered sex matching similar to the present study. However, a similar study on DH in fluorosed and non-fluorosed population has not considered sex matching.27
The distribution of DH was not significant between males (55.9%) and females (65.7%) within the FL group. (x2 = 1.9, p = 0.18, NS). Tonguc MO et al, reported higher frequency of DH in females (68.5%) compared to males (31.4%) in fluorosed population. In the NFL group, DH was significantly higher in females 51.0% as compared to males 36.0%. DH was significantly higher in both males and females in FL group compared to NFL group. (p <0.001, HS). A number of studies have reported higher incidence of DH in females than in males.16,18 Hasanain Al‑Khafaji, reported that the ratio of females to males with hypersensitivity was 1.3:1; which was statistically not significant. The findings of this study were similar to the present study. The possible reasons for the higher DH in females could be heightened oral hygiene awareness in women. The diets of females also differ. Consequently, patterns of abrasion and erosion of dentine may differ between sexes.16
Mild to moderate pain due to DH was observed in both F and NFL teeth which were significantly higher in FL teeth using both AS & TS methods. De Assis JS et al reported mean VRS scores of 1.7 ± 0.9 and 1.5 ± 0.8 in placebo group with AS & TS, respectively.28 Torwane NA et al, 29 reported 16% teeth with mild pain and 56% with moderate pain using AS in Group A, while 32% teeth had mild and 36% had moderate pain using TS in group A and stated that moderate dentin hypersensitivity was more prevalent than severe or mild varieties. Similar findings were reported by Kielbassa AM,30 and Tonguc MO et al,27 reported frequency of TS in the fluorosis group as 9.26%, which was significantly higher than the prevalence noted in the non-fluorosis group in their study (P=0.0003) and also higher than the DH prevalence of the general dental populations noted in other studies.31
Irrespective of the method used, 21.7% teeth had DH in the FL group compared to 11.1% in the NFL group. Chi-square analysis (x2 = 79.43; p < 0.001, HS) showed highly significant correlation between fluorosis and DH. Fluorosis a definiteetiology for DH. In the current study, the prevalence of DH in FL group was found to be 22.7%. Tonguc MO et al,27 reported frequency of tooth sensitivity using TS utilizing Williams periodontal probe & AS utilizing air blast from triple air syringe, in the fluorosis group as 9.26%, which was significantly higher than the prevalence noted in the non-fluorosis group in their study (P=0.0003) and also higher than the DH prevalence of the general dental populations noted in other studies.31 On the contrary Zhang Y, Sun G, Zheng Q reported that the prevalence of dental fluorosis in endemic fluorosis rural area was 95% and prevalence of dentine hypersensitivity evaluated using AS method utilizing air blast from triple air was 9.8%. In non endemic fluorosis rural area, the prevalence of dentinal hypersensitivity was 28.6%. It was concluded that the subjects in endemic fluorosis rural areas may have been suffering from dentine hypersensitivity less than the subjects in normal rural areas.
Recent research revealed that a systemic fluoride intake could have the opposite effect on dentin, making dentin more susceptible to dental caries and other defects such as tooth fractures. Dentin fluorosis has been found to distort the intertubular collagen network in dentin, thereby causing detrimental hypermineralization of dentin, resulting in a higher susceptibility to acid degradation.32 In addition, Rochas-Sanchez et al reported that the dentin in fluorotic teeth was characterized by a highly mineralized sclerotic pattern when compared to healthy teeth or fluorotic enamel lesions. In response to the effects of severe fluorosis in the enamel, the dentin showed hypermineralization, as seen in other enamel disorders. Furthermore, it was also shown that there was a positive correlation between the dentin fluoride concentration and the dentin tubule size, demonstrating wider dentin tubules in teeth with higher levels of fluoride in the dentin.14
On clinical examination, any tooth hypersensitive to either of the two methods (AS, TS) in the study was diagnosed with DH, as a few teeth which showed DH with TS method were not found with AS method. The severity of DH varied from nil (Score 0) to maximum (Score 50-75) in FL and NFL groups. Mild pain was found to be significantly higher than other VRS scores. As the severity of fluorosis increased from grade A to B, 3 (0.40%) teeth showed intense pain. Moreover, with increasing severity of fluorosis the percentage of teeth with dentinal hypersensitivity also increased. For absence of DH (VRS score 0) the maximum number of teeth (87.3%) and minimum number of teeth (60.3%) was found in Jackson’s scoring criteria B and F, respectively. For mild DH (VRS score 0-25), DH was observed in most of the FL teeth. The maximum number of teeth (38.4%) and minimum number of teeth (12%) was found in Jackson’s scoring criteria F and B, respectively. For Moderate DH (VRS score 25-50) maximum number of teeth (1.3%) and minimum number of teeth (0.0%) was found in Jackson’s scoring criteria F and C, respectively. For Intense DH (VRS scores 50-75), maximum number of teeth (0.4%) were found in Jackson’s scoring criteria B with no teeth showing DH in other FL grades. For Maximum DH (VRS score 75-100) none of teeth were found in any of the Jackson’s FL scoring criteria. Chi-square analysis (x2 =2.87; p <0.001, HS) showed a highly significant correlation between severity of fluorosis and severity of dentinal hypersensitivity in terms of VRS scores. It’s therefore clear that increased severity of fluorosis is directly related to increased severity of DH.
Tonguc MO et al, while comparing VAS scores of dentinal hypersensitivity in fluorosed and non-fluorosed subjects reported that the median and the most prevalent (79 teeth, 41.6%) Dean’s index score for the fluorosis group was 3 (min 1, max 4) and mean VAS scores 4.71±1.05 and 4.81±1.63 in FL & NFL groups respectively. However, no attempt was made to correlate severity of DH. Zhang Y, Sun G & Zheng Q, 2012 also conducted a similar study, however, the method used to assess the severity of DH is not known.
To the best of our knowledge, there are only two studies in literature related to DH in dental fluorosed subjects. Both the studies did not correlate the severity of DH with the severity of FL. Zero PI score was least (31.5%) in FL teeth than NFL (68.5%) teeth. The Plaque scores according to Turesky-Gilmore-Glickman modification of Quigley-Hein plaque index were found to be significantly higher in FL teeth. (p <0.001, HS)
The teeth affected by severe dental fluorosis suffer from post-eruptive enamel breakdown. The effect of fluoride on forming enamel results in a number of changes. These changes in the structure of enamel involve increased porosity, higher protein levels, and lower amounts of minerals and, in severe cases, the formation of a pitted surface.25 This presence of surface roughness can be one of the reasons for increased plaque scores in fluorosed teeth in the present study. On the contrary, Bozkurt FY and Tonguca MO et al, using Sliness & Loe plaque index reported lower PI scores in fluorosis group than non-fluorosis group. TMQH plaque index estimates the plaque on entire surface unlike other indices which may underestimate the plaque levels as it concentrates on cervical third of the tooth. It was found that plaque accumulation, gingival bleeding and inflammation were lower in subjects with fluorosis who were resident in Isparta compared to subjects with normal dentition who were resident in Konya, which is a non- fluorosis area in Turkey.33 Similarly, it was reported that as the concentration of fluoride in drinking water increased, plaque accumulation on tooth surfaces decreased.34 For this reason, although the enamel surfaces of fluorotic teeth have a high porosity, the amount of plaque deposited on these surfaces is lower than on nonfluorotic enamel surfaces.27 Moreover, it has been shown that high level of fluoride (at concentrations as high as 1000 ppm) in dentifrices reduces de novo plaque formation on tooth surfaces because high fluoride concentrations inhibit the metabolic and physiological pathways of biofilms. With fluoride concentration in drinking water which does not cause a similar effect on plaque formation due to shorter contact time and lack of retention on tooth surface.
The use of disclosing solution provided the better detection and scoring of plaque on tooth surfaces using TMQH index. There was highly significant correlation of plaque accumulation with DH occurrence (x2 = 8.15; p <0.001, HS; x2 = 12.56; p <0.001, HS) in both FL and NFL groups respectively. Intergroup comparison showed that a higher percentage (22%) of DH was found in FL than in NFL teeth (11%) [p <0.001, HS]. The role of plaque in inducing DH was obvious in FL teeth. Plaque accumulation on root surfaces may lead to demineralization of tooth structures which could be associated with patency of dentinal tubule orifices.(Kawasaki A et al, 2001) It has been reported that patients who maintain good levels of plaque control are less likely to report dentine hypersensitivity. (Manochehr-Pour M, 1984) On the other hand, patients who have significant proportions of their root surfaces covered with dental plaque report more problems with dentine hypersensitivity.35,36 Despite these findings, the influence of plaque on dentine hypersensitivity remains controversial. Interestingly, many patients with gingival recession have minimal plaque deposits yet still complain of sensitivity.37 Plaque accumulation, results in the subsequent production of acids within the plaque in response to glucose exposure. The introduction of dietary carbohydrate, even in thin layers of plaque, may result in a significant production of acid. This process of acid production is widely accepted to result in the demineralization of tooth structure (enamel and dentin) seen in caries which can lead to opening of dentinal tubules and thus DH.38
The tooth quality depends on mechanical and structural properties (Vieira APGF, et al, 2006) that are related to tooth sensitivity. Despite the caries preventive effectiveness of fluoride, it was found to have some negative effects on tooth quality.14 All tooth types were affected with DH. The type of FL teeth affected maximum with DH was Incisors 26.2% and minimum in Molars 16%. The order of tooth type affected with DH in FL group was: incisors>premolars>molars>canines. The type of NFL teeth affected maximum with DH was Molars 16.4% followed by minimum in Canines 5.5%. The order of tooth type affected with DH in NFL group was: Molars>incisors>premolars>canine. It was found that the teeth most commonly affected in general population where the fluorosis status was not known, were the premolars followed by first molars.12,16,39,40 However, few others reported lower anterior to be the most common teeth affected. There is increasing evidence41 to suggest dentine hypersensitivity may be a tooth wear phenomenon. Also the most common location of recession is on the facial aspect of canines, premolars, and molars,42,43 which can be the reason behind these teeth being the most sensitive.
However, in the current study molars were more commonly affected than premolars in FL group. Zhang Y, Sun G & Zheng Q reported that the commonest teeth affected with DH were the lower incisors and premolars in fluorosed and non-fluorosed population respectively. The findings of this are similar to our study. Tonguca MO et al reported that premolars were the most commonly affected teeth with DH. The grades of fluorosis of the teeth however have not been mentioned. The role of brushing habits in DH occurrence was studied in the current study. The tooth brushing once per day using medium brush in horizontal direction was found to be maximum in both FL and NFL groups. The use of medium brush in horizontal direction is traumatic to the dentition. Chrysanthakopoulos NA,44 reported that horizontal brushing method, usage of medium type toothbrush and tooth brushing once daily were found to be more associated with gingival recession, which is one of the etiological factors for DH. Traumatic tooth brushing in an healthy dentition would cause subclinical soft and hard tissue abrasion lesion that are most likely precursor of gingival recession, tooth wear and thus DH.37 The tooth brushing with and without tooth paste and an acidic diet probably link to both tooth wear and DH.41
This has led to the description of gingival recession/dentin hypersensitivity as “toothbrush disease.”37 This emphasizes that oral hygiene habit has important role to play also in the etiology of dentinal hypersensitivity and there is lack of oral health awareness in Indian population because of various socioeconomic, cultural and geopolitical reasons. The occurrence of DH in relation to GR is discussed as follows: GR was significantly higher in NFL than FL group. The type of GR according to Sullivans and Atkins differed between groups. This was similar to Tonguca MO et al, 2011who reported higher gingival recession in fluorosis group (1.31±0.4) than the non-fluorosis group (1.27±0.41). There was significant occurrence of DH of teeth within GR in NFL (36.5%) and FL (24%) groups. Intergroup comparison showed highly significant occurrence of DH of teeth with GR in NFL (60.1%) than FL (50.0%) groups. There is general consensus that gingival recession usually precedes dentin hypersensitivity and is perhaps the most significant predisposing condition of dentin hypersensitivity.45,46 Perhaps the most important factor in the etiology of dentin hypersensitivity is gingival recession because it causes exposure of root surfaces. Once gingival recession has exposed root surfaces, the cementum is rapidly lost from brushing with toothpaste and/or professional cleaning.38
The role of labioversion in DH causation is presented here. The presence of teeth with labioversion in FL (45.4%) and NFL (54.5%) was not significant. (p = 0.18, NS) Tooth malpositioning e.g. Crowding, orthodontic tooth movement inadvertently repositioning teeth outside the buccal plate, increases the risk of gingival recessions which in turn can lead to DH.37 These teeth are difficult to clean by the patient, increasing plaque accumulation further leading to attachment loss and recession. However, there are no reports in literature reporting frequency of DH in such teeth. In the current study, first attempt has been made to record Labioversion of teeth and occurrence of DH. A tooth with labioversion is prone for GR which in turn may lead to DH. Significantly higher percentage (36.5%) of teeth in labioversion with DH in NFL group compared to FL group.
The absence of wasting diseases (Attrition, Abrasion) was significantly higher (52.6%) in FL group as compared to NFL (47.4%). The presence of attrition (60.8%), Abrasion (81.3%) was significantly higher in NFL group. Erosion was absent in both the groups. Abfraction was observed only in a single tooth in FL group. Chi-square analysis (x2 = 59.6; p < 0.001, HS) showed significantly greater number of teeth with wasting diseases in the NFL group.
Once erupted into the oral cavity, fluorosed teeth are subject to chemical and physical injuries. The more hypomineralized the tooth is at time of eruption, the more likely it is to develop post-eruptive changes. As already discussed, the milder forms will be subject to surface attrition. In particular, occlusal surfaces are rapidly worn, often to such an extent that the hypomineralized porous layer is abraded away.47 In more severe cases, the hypomineralization is so extensive that the outermost well-mineralized surface layer is rather brittle and chewing forces may result in formation of surface enamel defects. Once the well-mineralized surface zone is fractured away, the exposed hypomineralized lesions are subject to extensive modifications by the oral environment. In the most severe cases, extensive abrasion of the very porous and soft exposed lesions occurs. The above results have been confirmed by SEM analysis48 of human fluorosed teeth which revealed highly uneven and rough surface. Cracks and fissures were also observed on it. The enamel surface showed pits of varying dimensions in the discoloured area of the teeth .The pits appeared to be lesions punched on the enamel surface, thus exposing the underlying porous enamel. A rougher surface as seen in fluorosed teeth can lead to greater plaque levels contributing further to DH.
The presence of DH in teeth with attrition (9.0% & 6.6%); abrasion (66.7% & 73.1%) were found respectively in FL and NFL teeth. The distribution of DH in FL and NFL teeth was not significant. (p >0.05, NS) Abrasion as a cause of DH was significantly higher than others (attrition, erosion, abfraction) Intergroup comparison using z test for proportions showed significantly higher 394 (22.7%) teeth without wasting diseases having DH in FL group compared to NFL group 119 (7.6%). (p = 0.04) Inetrgroup comparison for attrition, abrasion, erosion, abfration was not significant between FL & NFL teeth. (p> 0.05). Peripheral dentin is covered by cementum on root surfaces, and enamel on coronal surfaces. Thus, loss of enamel can expose dentin, placing it at risk of developing dentin hypersensitivity. The loss of enamel is usually a result of the combined actions of attrition, abrasion, and erosion. Two processes need to occur for dentine hypersensitivity to arise: dentine has to become exposed (lesion localisation), and the dentine tubule system has to be opened and be patent to the pulp (lesion initiation). Exposure of dentine may occur by loss of either enamel or periodontal tissues. The present available evidence suggests that lesion initiation in dentine hypersensitivity can be induced by abrasive and erosive agents, and mostly erosion alone is probably the more dominant factor, in synergy with abrasion, to bring about dentine wear and tubule opening.
Although the definition of dentinal hypersensitivity excludes the presence of any other dental defect or disease, it is an acknowledged fact that loss of enamel to expose dentinal tubule will entail some form of defect or disease in tooth. Essentially, dentine has to be exposed and the dentine tubule network opened to permit fluid movement under stimulation.49 There is increasing evidence41 to suggest dentine hypersensitivity may be a tooth wear phenomenon. It is now considered that erosion is a more important factor than abrasion in removing the smear layer or dentinal plugs thereby causing dentinal hypersensitivity. In addition, once erosion has occurred, patients are then more susceptible to subsequent abrasion, further exacerbating the loss of tooth structure and risk of dentinal hypersensitivity. Attrition can also be accelerated by the presence of softened tooth surfaces following erosion.
Hence, the results of this study suggest that subjects with dental fluorosis suffer from dentinal hypersensitivity more than subjects without fluorosis. Although the contribution of other etiologies of DH especially GR, Laioversion and wasting diseases to DH in fluorosed subjects needs to be assessed through multivariate regression analysis to come to a better conclusion. Improper tooth brushing habits can play a role in loss of soft and hard dental tissues leading to exposure of dentine and further leading to hypersensitive teeth, thus there is a need to educate the population regarding correction tooth brushing habits. Amongst the methods used to assess dentinal hypersensitivity air stimulation resulted in better identification of sensitive teeth, however since there were a few teeth diagnosed alone with tactile method, therefore, the use of at least two methods of DH assessment is highly recommended. Since fluorosis has global occurrence, studies have to be conducted from these areas, further studies with larger sample size need to be conducted to confirm the results of the present study.
Irrespective of the method used, the prevalence of DH in FL group was 21.7% which was significantly higher than 11.1% prevalence in the NFL group. A highly significant correlation was found between severity of fluorosis and severity of dentinal hypersensitivity in terms of VRS scores. The Plaque scores were found to be significantly higher in FL teeth compared to NFL teeth which could be due to surface roughness of fluorotic teeth which occur as a result of fluoride induced structural changes. The plaque levels in teeth with DH in FL and NFL groups were highly significant within both the groups indicating a confounding role of plaque in the etiology of DH especially in fluorosed teeth.
None.
Author declares that there is no conflict of interest.

©2020 Kour, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.