International Journal of
eISSN: 2573-2889


Research Article Volume 2 Issue 3
1Department of Biotechnology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Iran
3Department of Microbiology, Islamic Azad University, Tehran, Iran
Correspondence: Mojgan Bandehpour, Department of Biotechnology, Shahid Beheshti University of Medical Sciences, Iran
Received: March 14, 2017 | Published: May 3, 2017
Citation: Korani S, Kaszadeh M, Yarian F, et al. The expression of human tumor necrosis factor receptor (HTNFR) in the CHO cell line. Int J Mol Biol Open Access. 2017;2(3):94-97. DOI: 10.15406/ijmboa.2017.02.00021
Tumor necrosis factor (TNF) is a multifunctional Proinflammatory cytokine that stimulates acute phase reactions by acting on TNFR1 and TNFR2 receptors. Deregulation of TNF production can lead to various uncontrolled inflammation diseases. One of the medical applications of TNF receptors (TNFR) is the treatment of inflammatory diseases such as rheumatoid arthritis (RA). The aim of the present research was to express TNFR1 in the eukaryotic system. Synthesized gene encoding the TNFR1 into pGH vector was sub cloned into the pEGFP-N1 plasmid and transfected into the CHO-K1 cell line. Purification of the recombinant TNFR was carried out by affinity chromatography. Green fluorescence in the cell culture confirmed the expressed protein and it was shown a sharp band in the western blot analysis. So CHO-K1 is able to express recombinant TNFR1 properly in lab scale conditions.
Keywords: purification, TNFR, pegfp-n1, affinity chromatography
Cytokines, including necrosis factor, play an important role in inducing and limiting inflammation. Cytokines play a pivotal role in coordinating the negative and positive signals required to produce and shape inflammatory responses. TNF and its receptors act as a well-known Proinflammatory cytokine system and they are important in various biological processes.1‒3 The tumor necrosis factor receptor superfamily (TNFRSF) is named based on the first member of this family, that is, tumor necrosis factor (TNF), and it already has more than 40members with structural similarities. TNF leads to cytolysis of many tumor cells in vitro and has been found to cause hemorrhagic necrosis of transplanted tumors in rats, enhancing phagocytosis and the cytotoxicity of polymorpha nuclear neutrophils, as well as modulating the expression of many proteins, including lipoprotein lipase, class I antigens associated with major histo compatibility complex (MHC), and cytokines such as interleukin-1 and interleukin-6.4 TNF is a membrane-bound homodimer protein that can be released by proteases in the form of soluble cytokine. Both the membrane and the soluble cytokines are biologically active. It is produced by monocytes and macrophages, dendritic cells, B cells, T cells, and fibroblasts, as well as by fat cells, keratinocytes, breast, and colon epithelial cells, osteoblasts, mast cells, and many other cell types3,5 (TNF acts through two distinct receptors. NFR1 (also designated as p55) and TNFR2 are also known as P75. In addition, both receptors can be cleaved at the cell surface and changed to a soluble form, and from here, they can serve as decoy receptors (i.e., no signaling receptors) for the TNF function6,7 (The dysregulation of TNF production can lead to uncontrolled inflammation and various diseases such as RA, which is characterized by chronic inflammation and bone erosion. Autoantibodies, cytokines, and chemokine are possible RA triggers. TNF receptors are used as therapeutic agents for the inhibition of cytokines according to their characteristics (targeting certain cytokines), high affinity (an affinity usually comparable to or greater than many existing anti-cytokine monoclonal antibodies), and lack of immunogenicity (unlike using rat mAbs used to treat humans, these receptors do not cause an antibody response in humans)8 (We hope that the recombinant TNF-alpha receptor will help in the treatment and control of inflammatory diseases. This study aims to investigate TNFR gene cloning and the expression of recombinant protein in eukaryotic cells, as well as purifying TNFR to be used in future research for the treatment of inflammatory diseases.
1-Synthesis of the TNFR gene in pGH vector
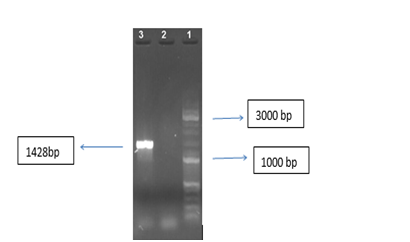
The TNF receptor gene was selected based on the sequences in the Gen Bank (Gene ID: 7133). It was synthesized into pGH plasmid (Bioneer, Korea). Cloning accuracy was confirmed using a polymerase chain reaction (PCR).9 The primers designed to amplify the TNFR gene are as follows:
These primers have the sequences required to identify and cut with the BamHI and NheI enzymes (Fermentas, Lithuania).
2-Subcloning of the TNFR gene in the eukaryotic pEGFP-N1 expression vector
The pGH vector containing TNFR gene and the pEGFP_N1 expression vector were digested with BamHI (Fermentas, Lithuania) and NheI enzymes. After purification of TNFR gene and the digested pEGFP_N1 vector from agarose gel using the gel purification kit (Bioneer, Korea), ligation was carried out with T4 DNA Ligase (Fermentas, Lithuania). The recombinant plasmids were confirmed by colony PCR and restriction enzyme analysis.
Transfection of the recombinant vector in the CHO cell line using a calcium chloride buffer. CHO cell line was cultured routinely in the DMEM culture medium containing 10% fetal calf serum (FCS), 100U/ml penicillin, and 100µg /ml streptomycin at 37°C 5% CO2. The cells were separated by Trypsin 24hours before transfection. When the cell density reached 60–70%, transfection was carried out with the pEGFP_TNFR plasmid. Three hours before transfection, the cell culture medium was replaced with serum-free DMEM. Two solutions were prepared in two separate tubes: 1 x HBS (solution 1) and a solution containing 9µg of DNA and calcium chloride (2M) (solution 2). Solution 2 was added to Solution 1 and the resulting solution was incubated at room temperature for 20minutes and added gradually to the cell flask. Following incubation for 16hours, the culture medium was replaced with the neomycin DMEM. Then, 40hours later, TNF receptor gene expression was analyzed by fluorescent microscopy.
3-SDS-PAGE and western blotting
The quality of the soluble expressed TNFR protein was confirmed by SDS-PAGE and Western blotting.10 After dialysis and precipitation of secreted protein in the culture medium, it was subjected to SDS-PAGE on a 12% polyacrylamide gel. The samples were mixed with a 2X loading buffer (125mM Tris, 20% Glycerol, 4% SDS and 0.01% bromophenol blue at pH 6.8). Gel staining was carried out with Coomassie brilliant blue R-250. For western blotting, the recombinant proteins were separated on a 12% polyacrylamide gel and were electrophoretically transferred to a nitrocellulose membrane (Whatman, UK). The transblotted membrane was blocked for 2h at room temperature with a blocking solution (3% (w/v) skimmed milk in TBS (Tris-Buffered Saline) (Sigma, USA). The membrane was incubated for 2h at room temperature with the anti-TNFR (Abcam, UK) 1/5000 dilution monoclonal antibody. After washing, the membrane was incubated for 2h at room temperature with secondary alkaline phosphatase conjugated anti-mouse antibody (Abcam, UK). After three times washing for 5min with TBST, the NBT/BCIP substrate solution (Roche, Germany) was used to visualize the immunoreactivity.
4-Purification of TNFR using Ni-NTA His Tag affinity Chromatography
The recombinant His-tagged TNFR protein was purified via affinity chromatography using Ni2+-NTA agarose resin (Novagen, USA). After dialysis of cell culture medium with equilibration buffer (20mM NaH2PO4, and 500mM NaCl at pH 8.0) it was loaded on an equilibrated resin. Three times washing of the resin was performed with 10ml of wash buffer (10mM, 20mM, 30mM and 40mM gradient of imidazole, 20mM NaH2PO4 and 500mM NaCl at pH 8.0), the bounded protein were eluted with 1 ml of elution buffer (300mM imidazole, 20mM NaH2PO4 and 500mM NaCl at pH 8.0).11 Eluted protein was dialyzed in PBS at room temperature for 2h.
1-Amplification of the TNFR gene:
The TNFR gene was amplified with specific primers as shown in Figure 1.
2-Expression of the TNFR protein in CHO cell line
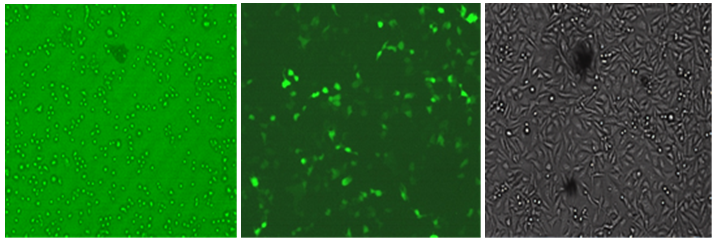
The recombinant pEGFP-N1-TNFR vector was confirmed with the specific primers. The his-tagged TNFR was expressed and purified via affinity chromatography. It was analyzed using SDS-PAGE. The TNFR protein band with 53kDa in size is observed in Figure 2 and illustrates the results of the Western blot analysis of the purified protein. Transfection of the recombinant pEGFP-N1 / TNFR vector in the CHO cells was performed using a calcium chloride buffer method. After 40hours, green fluorescent light in the desired positive cell line control sample containing pEGFP-N1 and transfected cell line with recombinant plasmid was observed Figure 3.
In the present study, a eukaryotic system was used to express the recombinant protein of the TNF-alpha receptor. For this purpose, approved procedures were performed to clone the gene encoding this receptor into the pEGFP-N1 expression vector, while the CHO eukaryotic cell line was used to express the recombinant protein.12 In addition, due to the high expression of this plasmid and the fact that it is fluorescent with more production and more comfortable tracking during the expression stage were possible.13 The reason for choosing this receptor was the vital role of the TNF cytokine as a powerful inflammatory cytokine that is considered a strong mediator in inflammation and immune response. This receptor’s expression level is very important because its overexpression leads to autoimmune diseases such as Rheumatoid Arthritis and tissue damage.14 TNF acts via two receptors, namely, TNFR1 and TNFR2. TNFR2 is expressed by immune and endothelial cells. TNFα antagonists are already among the most commonly used methods for clinical treatment.3,15 As a therapeutic agent for inhibiting the activity of TNFα, TNFR has several advantages over some of the medications that suppress the immune system. Our research findings showed that CHO-K1 and the pEGFP-N1 vector are the appropriate system for the production of recombinant TNFR as drug protein whereas according to the Joshua A. Bornhorst and Joseph J. Falke, the protein can accept a polyhistidine affinity tag, and up to 95% purities with a 90% recovery of tagged protein can be achieved in a single purification step by purifying the Ni-NTA column of highly expressed protein.16 Also some researches were performed on the coding regions of receptors (TNFR1 and TNFR2) were integrated with the mammalian expression vector pRK5. Lewis et al. applied the TSA 201 cell line as a sub cloned off the human embryonic kidney cell line to detect the expression of the TNFRs proteins, as well as the lipofectamine technique to insert the recombinant plasmid into TSA 201 cells.17
Due to the side effects of the drugs used to treat some diseases, investing in such medications and optimizing them will be of significant economic and scientific value. Therefore, the production of TNFα receptor in the CHO cell line and of E.coli bacteria to produce more of that receptor to bind and block TNFα and prevent the inflammatory response in diseases such as Rheumatoid Arthritis, as well as to provide pain relief, is of great importance.
One of the benefits of this study, and the main difference between it and other researches is the application of the pEGFP-N1 vector to clone this gene. A primary characteristic of this vector is its expressivity in the eukaryotic system and the fact that it has green fluorescent protein (GFP), whose presence in this vector made it easier to identify and track the vector in our expression cell.13,18 Other distinguishing aspects of the project include the use of CHO cells to express the TNFα receptor gene.
Based on the aforementioned features and the experience gained over the past two decades, as well as World Health Organization safety tests, therapeutic proteins produced by this cell line have been confirmed without any problems, so that today, annual sales of products produced by CHO cells amount to more than $30billion worldwide19 . As noted above, one of the benefits of CHO cells is their ability to produce glycosylated proteins, and some of the benefits of protein glycation are as follows: - It increases the duration of the action of proteins. Sugar entering the protein causes a certain conformation of the protein, and this allows easier identification by cell surface agents and therefore facilitates protein entry into the cell. Different plasmids have been used for cloning the TNF receptor gene. Many of these either have improper expression or have the proper expression but are of the viral type. Due to the toxicity of viral plasmids, along with ethical aspects, the use of non-viral plasmids seems to be safe in treatments compared to virus transmission systems. Accordingly, in this study, a non-viral vector was selected. The results will help us achieve the objectives of protein testing on animals, in addition to mass production of these proteins in the Ian as medicines. This, in turn, will allow us to compare the effect of the recombinant protein with common medicines in which proteins are purified as a result of gene expression.
This work was performed in the Cellular & Molecular Biology Research Center of Shahid Beheshti University of Medical Sciences. This article was extracted from Shahla Korani's Ph.D. thesis. This study was funded by the deputy of Shahid Beheshti University of Medical Sciences (grant number 5061) and consulted to the doing of the experiment with the ethics code IR.SBMU.RAM.REC.1394.82.
Author declares that there is no conflict of interest.

©2017 Korani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.