International Journal of
eISSN: 2573-2889


Opinion Volume 2 Issue 2
1Centre of Excellence in Alzheimer’s disease Research and Care, School of Medical and Health Sciences, Edith Cowan University, 270 Joondalup Drive, Joondalup, 6027, Australia
2School of Psychiatry and Clinical Neurosciences, The University of Western Australia, Nedlands, 6009
3Mc Cusker Alzheimer’s Research Foundation, Hollywood Medical Centre, 85 Monash Avenue, Suite 22, Nedlands, 6009, Australia
Correspondence: Martins, School of Medical Sciences, Edith Cowan University, 270 Joondalup Drive, Joondalup, Western Australia 6027, Australia, Tel 61 863042574
Received: February 24, 2017 | Published: March 2, 2017
Citation: Ian M. The future of genomic medicine involves the maintenance of sirtuin 1 in global populations. Int J Mol Biol Open Access. 2017;2(2):42-45. DOI: 10.15406/ijmboa.2017.02.00013
Genomic healthcare now requires the need for the critical assessment of novel molecular biological discoveries that may identify anti-aging genes and transcription factors that are defective in non alcoholic fatty liver disease (NAFLD), obesity, diabetes, cardiovascular disease and neurodegenerative diseases. It is estimated that 30% of the global community will be at risk for NAFLD and the future of genomics in medicine may require nutrigenomics in shaping medicine with fundamental shifts in how we define the nutritional treatment of global chronic disease. The Sirtuin 1 (Sirt 1) gene has now been identified as the gene to be defective and linked to genetic diseases, NAFLD, diabetes and neurodegenerative diseases. Bacterial lipopolysaccharide (LPS) may induce various chronic diseases and may act as a competitive inhibitor to Sirt 1 with glucose and cholesterol toxicity to various cells and tissues. Nutritional diets are required to activate Sirt 1 and improve drug response to cells with increased hepatic drug metabolism important to prevent drug induced toxicity with relevance to the global chronic disease epidemic.
Keywords: genomic, medicine, global, sirtuin 1, nutrigenomics, nafld, obesity, diabetes, bacterial, lipopolysaccharide, transcriptional, dysregulation, fibroblast growth factor 21
Genomic medicine has become of importance to the current global chronic disease epidemic. Genomic healthcare will become of critical importance when genomic information can be used routinely to improve the health, diagnosis and treatment of all individuals.1 Genomic medicine has been used for next-generation sequencing in cancer pharmacogenomics for the diagnosis of rare disorders and determination of infectious disease outbreaks. The progress in dissecting the molecular basis of common diseases such as the role of the host microbiome, identification of drug response biomarkers and determination of hepatic drug metabolism has become important.2 The difficulty in establishing clinical validity and utility of tests to increase their awareness and promote their use for genomic healthcare now require the need for the critical assessment of novel molecular biological discoveries that include the anti-aging genes and transcription factors that become defective early in life with relevance to non alcoholic fatty liver disease (NAFLD), obesity, diabetes, cardiovascular disease and neurodegenerative diseases. In the year 2015 it is now estimated that 30% of the Western World will now progress to NAFLD and by the year 2050 if NAFLD remains untreated in the Western world the prevalence of the disease may rise to 40% of the global population.3 The future of genomics in medicine may require nutrigenomics in shaping medicine with fundamental shifts in how we define the nutritional treatment of global chronic disease,4 (Figure 1).
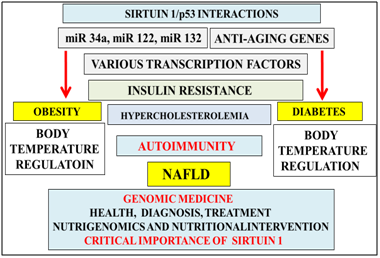
Figure 1 The anti-aging gene Sirt 1 is defective in obesity and diabetes with miRNAs relevant to Sirt 1 repression. Nutrigenomics is now important to genomic medicine with activation of Sirt 1/p53 interactions important to transcriptional dysregulation of cell glucose and cholesterol metabolism. Defective Sirt 1 activity in cells may be associated with insulin resistance and autoimmune disease and connected to programmed cell death in various tissues/organs. Nutritional interventions in individuals with chronic diseases may be ineffective with relevance to thermoregulation defects in individuals in the global chronic disease epidemic.
The gene-environment interaction in Western countries indicates that with urbanization5 and access to food its content may lead to induction of epigenetic alterations that are associated with lipid and glucose dyshomeostasis with increased risk for insulin resistance relevant to Type 1, Type 2 and Type 3 diabetes and obesity (Figure 1) in these countries. The gene environment interaction now identify the nuclear receptor Sirtuin 1 (Sirt 1) that regulates appetite6 and neuron proliferation7 to be involved in the induction of insulin resistance that involve alterations in nuclear receptors, micro RNA with chromatin remodelling8 that are now closely associated with global chronic disease (Figure 1). The interest in glucose metabolism has accelerated with the role of Sirt 1 and its role in the transcriptional regulation of p539 linked to p53 transcriptional regulation of caveolin 1 expression 10‒12 associated with insulin receptor transport and activity.13‒16 Sirt 1’s involvement in cholesterol transport requires the nuclear liver X receptor-ATP binding cassette transporter protein 1 (LXR-ABCA1) and Sirt 1 mediated caveolin expression involved in cholesterol metabolism in the liver and brain.4
Sirt 1 is a nicotinamide adenine dinucleotide (NAD+) dependent class III histone deacetylase (HDAC) that targets transcription factors to adapt gene expression to metabolic activity and the deacetylation of nuclear receptors indicate its critical involvement in insulin resistance.8,9 Sirt 1 is also involved in telomerase reverse transcriptase and genomic DNA repair with its involvement in telomere maintenance that maintains chromosome stability and cell proliferation.17,18 In situ hybridization analysis has localized the human Sirt1 gene to chromosome 10q21.3.19 The Sirt 1 gene has now been linked to various diseases with deletions, inversions and aberrations in chromosome 10q21.3.20‒25
Sirt 1 targets transcription factors peroxisome proliferator-activated receptor-gamma coactivator (PGC-1 alpha), p53, pregnane x receptor (PXR) by deacetylation to adapt gene expression to mitochondrial biogenesis with effects on metabolic activity, insulin resistance and inflammation.8 Furthermore Sirt 1/p53 interactions may regulate adipocytokines and immunometabolism that may be important to NAFLD, obesity and neurodegeneration.26 Over nutrition is associated with the repression of Sirt 1/p53 interactions and other anti-aging genes such as Klotho, p66shc (longevity protein) and FOXO1/FOXO3a6 that are now connected to autonomous diseases of the brain and liver.
Dietary fat down regulation of Sirt1contributes to reduced adiponectin expression in obesity and diabetes27 with effects on adipose tissue transformation and development of liver disease.28 Sirt 1 increases adiponectin transcription in Adipocytes by activation of forkhead transcription factorO1(Foxo) interaction with CCAAT/enhancer binding protein alpha(C/EBPalpha) to form a transcription complexat the mouse adiponectin promoter that up-regulates adiponectin gene transcription.27 Sirt1 interactions with C/EBP alpha may involve Klotho C/EBP alpha and peroxisome proliferator-activated receptor (PPAR) interactions29‒31 with their important triolein Adipocyte differentiation. Micro RNA (miR) such as miR-34a, miR-122 and miR-132 are associated with Sirt 1 repression relevant to low adipose tissue adiponectin release and the development of metabolic disease and NAFLD.8
NutritionandPPARalpha-Sirt1 expression is related to hepatic Fibro blast growth factor21 (FGF21) production important to NAFLD and diabetes.32‒37 FGF21is an important activator of Sirt1mediated release of adiponectin.38 FGF21 binds to FGF receptor and beta klotho receptor complex38‒43 and activates adipose tissue Sirt1 by increases in NAD+ and activation ofPGC1-alpha and AMP activated protein kinase (AMPK).37,44 FGF21 and its effect on thermoregulation45 may involve Sirt 1 regulation of heat shock proteins (HSP) by deacetylation of heat shock factor (HSF) via PGC1α as a critical repressor of HSF1-mediated transcriptional programs.46,47 Sirt 1 is involved in body temperature regulation of the mammalian target of rapamycin (mTOR) signaling through the tumor suppressor tuberous sclerosis complex 1 with relevance to the expression of hepatic PGC-1α and FGF21.48
Genomic medicine is delivered to patients and members of the general global community in managing their own health with interventions critical to prevent bacterial lipopolysaccharide (LPS) that are involved transactivation of p53 and repression of Sirt 1 associated with defective nuclear and mitochondria interactions and the induction of various chronic diseases,8 (Figure 2). Plasma LPS levels have increased in the developing world with LPS effects on caveolin expression and the LXR-ABCA1 pathway4 that induce hypercholesterolemia and NAFLD.49 LPS repression of Sirt 1 also involves neutralization of apolipoprotein E49 with relevance to corruption of peripheral cholesterol, alpha synuclein and amyloid beta metabolism50,51 relevant to inflammation, cardiovascular and neurodegenerative diseases.
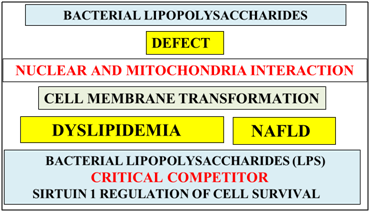
Figure 2 Bacterial lipo polysaccharides may act as a competitive inhibitor to Sirt 1 and prevent its role in the survival of cells in many diseased tissues. LPS may induce cell membrane transformation with the induction of dyslipidemia and NAFLD. The major defect in diseased cells from the global chronic disease epidemic is the defective nuclear-mitochondria interaction with relevance of LPS to increased toxicity and autonomous organ disease.
LPS inserts itself into the cell membrane by binding to the cholesterol/sphingomylein domain with transformation of the cell membrane that involves GM1 ganglioside relevant to various chronic diseases (Figure 2).52 LPS effects on Sirt 1 repression are important to increased toxic heat shock proteins and defective thermoregulation associated with autoimmunity that involve the natural killer cells and macrophages.26,48,53 LPS induce zinc and magnesium deficiency50,54 both activators of Sirt 1with corruption of Sirt 1’s role in genomic medicine with the development of autonomous organ diseases. LPS may be referred to as a competitive inhibitor of Sirt 1’s role in the regulation of cell cholesterol and glucose metabolism critical to maintain organ/tissue survival (Figure 2).
This work was supported by grants from Edith Cowan University, the Mc Cusker Alzheimer's Research Foundation and the National Health and Medical Research Council.
Author declares that there is no conflict of interest.

©2017 Ian. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.