International Journal of
eISSN: 2573-2889


Research Article Volume 1 Issue 1
1Department of Zoology, Mizoram University, India
2Department of Biotechnology, India
Correspondence: Ganesh Chandra Jagetia, Professor and Head, Department of Zoology, Mizoram University, Mizoram, India, Tel 011-389-2330724
Received: October 29, 2016 | Published: December 28, 2016
Citation: Jagetia GC, Reddy TK. The grape fruit bioflavonoid naringin protects against the doxorubicin-induced micronuclei formation in mouse bone marrow. Int J Mol Biol Open Access. 2016;1(1):48-55. DOI: 10.15406/ijmboa.2016.01.00006
Doxorubicin (DOX) is clinically used in the management of numerous neoplastic disorders however; development of life threatening cardiotoxicity and normal cytotoxicity is a major impediment in realizing its full potential in cancer therapy. The effect of various doses of Naringin was investigated on the DOX-induced DNA damage by micronucleus assay in mice bone marrow. Administration of mice with 5, 10 or 15 mg/kg body weight of DOX resulted in a dose dependent rise in the frequency of micro nucleated polychromatic (MPCE) and 0 chromatic (MNCE) erythrocytes accompanied by a DOX-dose related decline in the polychromatic erythrocytes and 0 chromatic erythrocytes (PCE/NCE) ratio. The frequencies of MPCEs and MNCEs increased with scoring time and the greatest rise in MPCE was observed at 48 h post-DOX treatment, whereas a maximum increase in MNCE was recorded at 72 h post-DOX treatment. The PCE/NCE ratio declined continuously with scoring time with a maximum decline at 72 h post-treatment. Mice administered with 2.5, 5, 7.5 and 10 mg/kg body weight of Naringin orally once daily for 5 consecutive days before DOX-treatment showed a significant attrition in the DOX-induced micronuclei frequency accompanied by a significant rise in the PCE/NCE ratio at all scoring times when compared to DOX treatment alone. The greatest protection against DOX-induced genotoxicity was observed for 10 mg/kg Naringin. Naringin reduced the DOX-induced DNA damage as indicated by a significant decline in the micronuclei formation. This which may be due to inhibition of free radicals, increased antioxidant status, metal chelation and restoration of topoisomerase-II activity by Naringin.
Keywords: doxorubicin, naringin, chemo protection, micronuclei, bone marrow and mouse
DMEM, dulbecco’s modified eagle’s medium; FCS, fetal calf serum; DDW, double distilled water; PCE, polychromatic erythrocytes; DOX, doxorubicin; B. Wt, body weight; NIN, naringin
Doxorubicin (DOX), an anthracycline group of antitumor antibiotics was isolated from Streptomyces peucetius in Italy.1 It is a widely used cancer chemotherapeutic agent, which is active against a wide variety of neoplastic disorders including solid tumours, various hematological malignancies, breast, bile duct, prostate, uterus, ovary, oesophagus, stomach, hepatic and childhood solid tumors. It is also active against osteosarcomas, soft tissue sarcomas, Kaposi’s sarcoma, Wilm’s tumor and myeloblastic and lymphoblastic leukaemias.2‒3 The clinical use of DOX is a double-edged sword; on the one hand it is an important part of chemotherapeutic regimens in the management of several neoplastic diseases, whereas on the other hand its chronic administration induces severe cardiomyopathy and congestive heart failure.3–5 The DOX is known to induce severe bone marrow suppression, and its long-term clinical use is limited due to the cumulative dose-dependent irreversible chronic cardiotoxicity, which manifests itself as congestive heart failure.6‒8
A large body of evidence indicates that the dominant cellular target of DOX is cellular DNA.9‒12 DOX is known to cause single strand DNA breaks, chromosomal rearrangements and mutational events, and it is a potent carcinogen.13 DOX has been reported to induce micronuclei, chromatid and chromosome aberrations, DNA single and double strand breaks in vitro and in vivo.14‒22 Therefore, it is essential to screen pharmacological agents, which can reduce the DOX-induced cumulative toxicity and genotoxicity in normal cells. The toxic effects of chemotherapeutic agents are generally reduced clinically by prescribing vitamins or vitamin supplements. Flavonoids are a class of naturally occurring compounds with excellent iron chelating and radical scavenging properties 22‒28 and are therefore of interest as possible modulators of DOX-induced genotoxicity. Naringin (NIN), present in most of the citrus species, has metal-chelating, antioxidant, anticancer, hepatoprotective, antidyslipidemic, neuroprotective and free radical scavenging properties and may offer some protection against mutagenesis.28‒34 Naringin, at low dose has been found to inhibit lipid peroxidation.35 It has also been reported to reduce lipopolysaccharide induced tumor necrosis factor,36 and H2O2-induced cytotoxicity, apoptosis and genotoxicity.37 Naringin, has been reported to protect cells against the radiation-induced chromosome damage, iron-induced oxidative stress, and bleomycin-induced genotoxicity22‒29 as well as cytosine arabinoside (Ara-C)-induced cytotoxicity.38 Treatment of mice with Naringin, has also been reported to reduce benzo- a- pyrene-induced tumor burden and micronuclei induction.13 It has also been found to protect against the doxorubicin induced cardiotoxicity without affecting the antitumor activity of the latter.13 Naringin has been reported to protect bleomycin induced lung fibrosis and doxorubicin-induced caridotoxicity in rats.39,40 Naringin has been reported to be nontoxic up to 16g kg. b. wt. in acute toxicity studies in rats and it also did not induce any adverse side effects when administered at a dose of 1250mg/kg/day for 13weeks in rats.41 The systematic evaluation of the effect of Naringin on DOX-induced genotoxicity/clastogenicity is lacking. Therefore, present study was designed to evaluate the protective effect of Naringin, on DOX-induced genotoxicity in the bone marrow cells of Swiss albino mice receiving different doses of DOX.
Chemicals
Naringin was procured from Acros Organics Ltd., Geel, Belgium, whereas doxorubicin hydrochloride was purchased from the Biochem Pharmaceutical Industries, Mumbai, India. Acridine orange, fetal calf serum (FCS), Dulbecco’s modified Eagle’s medium (DMEM) and other chemicals were obtained from Sigma Aldrich Chemical Company, Bangalore, India.
Animal care and handling
The animal care and handling were carried out according to the guidelines issued by the World Health Organization and INSA, New Delhi, India regulations. The study was approved by the Institutional Animal Ethical Committee of the Kasturba Medical College, Manipal, India and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Ten to twelve weeks old male Swiss albino mice weighing 30 to 36 g were selected from an inbred colony maintained under the controlled conditions of temperature (23±2 ºC), humidity (50±5%) and light (12 h of light and dark, respectively). The animals had free access to sterile food and water. Four animals were housed in a polypropylene cage containing sterile paddy husk (procured locally) as bedding throughout the experiment.
Preparation of drug solution & mode of administration
Naringin (NIN) or doxorubicin hydrochloride (DOX) was dissolved in sterile double distilled water (DDW), immediately before use. The animals were injected either with 0.01 ml/g body weight (b. wt.) DDW or various doses of DOX intraperitoneally, whereas NIN was administered using an oral gavage (Popper & Sons, Inc., New Hyde Park, NY, USA) once daily for five consecutive days before DOX treatment.
The chemoprotective effect of Naringin was studied in male Swiss albino mice, which were divided into the following groups:-
Bone marrow micronucleus assay
The animals from all the above groups were killed by cervical dislocation at 12, 24, 48 or 72 h after DOX-treatment. The micronuclei (MN) were prepared according to the method of Schmid with certain modifications described by Jagetia et al. 11 . Briefly, the femurs of each animal were dissected out, cleaned and the bone marrow was flushed out into DMEM individually. The cells were pelleted by centrifugation. A few drops of (FCS) were added and the pellet was thoroughly mixed. The smears were drawn on to precleaned coded slides using a drop of the resultant suspension in FCS. The slides were air dried and fixed in absolute methanol. The slides were stained with 0.05% Acridine orange in Sorensen’s buffer (pH 6.8) and washed twice in Sorensen’s buffer. The buffer mounted slides were observed under a fluorescence microscope (Carl Zeiss Photomicroscope III, Oberkochen, Germany) using a 40 x Neofluar objective. A minimum of 1000 each (PCE) and (NCE) were screened for the presence of MN for each animal for each dose of DOX or NIN. A total of 4000 each PCE or NCE were scored for each dose of NIN as well as DOX for each group. Data regarding the polychromatic and normochromatic erythrocyte ratio (PCE/NCE ratio) were also collected, where a minimum of 4000 erythrocytes per animal was scored. Four animals were used for each dose of DOX, as well NIN for each group at each scoring time and a total of 332 animals were used to complete the whole experiment.
The protection factor (PF) was calculated using the following formula:
The statistical significance among the treatments was determined using one-way analysis of variance (ANOVA). Bonferroni’s post-hoc test was applied for multiple comparisons, wherever necessary. The data were fitted on to linear (Y = α + βD) or linear quadratic (Y = C + αD + βD2) equations to describe the dose response, if any, where C is control MN frequency, D is DOX dose and α & β are the constants.
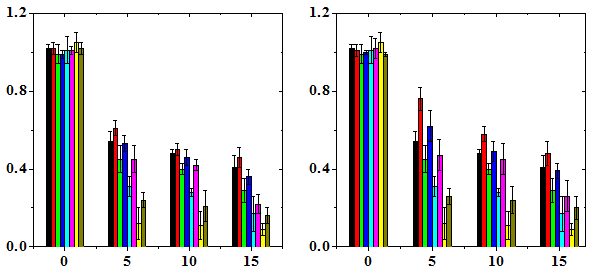
The results are expressed as micronucleated polychromatic erythrocytes (MPCE) or micronucleated normochromatic erythrocytes (MNCE) per 1000 ± SEM (standard error of the mean) and PCE/NCE ratio ± SEM (Table 1-3 & Figures 1-3). Administration of various doses of NIN alone did not alter the spontaneous frequency of micro nucleated polychromatic erythrocytes significantly (Table 1). Treatment of mice with different doses of DOX caused a significant but dose dependent rise in the MPCE frequency at all post-scoring times studied with a highest elevation for 15 mg/kg DOX (Figure 1). The frequency of MPCE increased with increasing scoring time and a maximum increase in MPCE frequency was scored at 48 h post treatment for all DOX doses. Thereafter MPCE registered an abrupt decline at 72 h and it was approximately 1.8 to 2.6 fold lower than that of 24 h depending on the DOX dose (Table 1). Treatment of mice with various doses of DOX also induced MPCE bearing two micronuclei at all post-treatment times and their frequencies were highest at 48 h post-treatment, which declined thereafter (Figure 1). This increase in MPCE was approximately 4 (p<0.001), 10 (p<0.001) and 13 (p<0.001) folds, at 12 h, 8 (p<0.001), 17 (p<0.001), and 19 (p<0.001) folds, at 24 h, 17 (p<0.001), 30 (p<0.001), and 37 (p<0.001), folds, at 48 h and 9 (p<0.001), 17 (p<0.001), and 14 (p<0.001) folds, at 72 h, for 5, 10 and 15 mg/kg DOX, respectively when compared with the non DOX-treated control (Table 1). The pattern of increase in MPCE in NIN+DOX group was identical to that of DOX group except that the treatment of mice with 2.5, 5, 7.5 and 10 mg/kg b. wt. NIN before administration of various doses of DOX caused a significant but NIN dose dependent reduction in the frequency of MPCE depending on the scoring time (Table 1). A greatest reduction in MPCE was observed for 10 mg/kg NIN. The protection factor depended on the dose of NIN and a highest PF of 2 was observed for 10 mg/kg NIN for all doses of DOX at 48 h post-treatment (Table 4). The frequency of PCE bearing two and multiple micronuclei revealed no specific pattern of induction except that at 48 h the MPCE with 2 MN showed a DOX-dose dependent elevation, whereas NIN treatment reduced the frequency of DOX-induced 2 MNMPCE in NIN dose dependent fashion (Table 1). The dose response was linear (Figure 1).
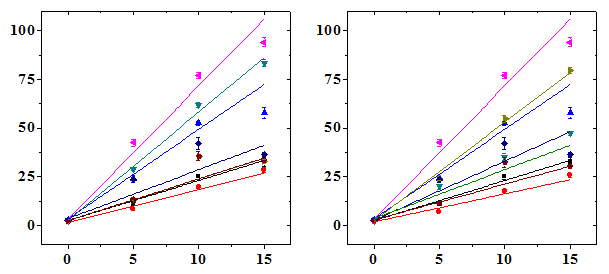
Micronucleated Ploychromatic Erythrocytes/1000
Doxorubicin (mg/kg b. wt.)
Treatment |
|
Micronucleated polychromatic erythrocytes (MPCE 1000 ±SEM) |
|
|
|
|
|
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DOX |
NIN |
12 h |
|
|
24h |
|
|
48h |
|
|
72h |
|
|
mg/kg |
mg/kg |
One |
Two |
Total |
One |
Two |
Total |
One |
Two |
Total |
One |
Two |
Total |
0 |
0 |
2.50±0.5 |
0.0±0.0 |
2.50±0.5 |
3.00±0.5 |
0.0±0.0 |
3.0±0.5 |
2.25±0.2 |
0.25±0.0 |
2.50±0.5 |
2.50±0.5 |
0.0±0.0 |
2.50±0.5 |
2.5 |
2.34±0.4 |
0.0±0.0 |
2.34±0.4 |
2.76±0.29 |
0.0±0.0 |
2.76±0.29 |
2.37±0.16 |
0.0±0.0 |
2.37±0.16 |
2.25±0.46 |
0.0±0.0 |
2.25±0.46 |
|
5 |
2.4±0.31 |
0.0±0.0 |
2.4±0.31 |
2.31±0.41 |
0.0±0.0 |
2.31±0.41 |
2.42±0.31 |
0.0±0.0 |
2.42±0.31 |
2.76±0.31 |
0.0±0.0 |
2.76±0.31 |
|
7.5 |
1.98±0.44 |
0.0±0.0 |
1.98±0.44 |
2.45±0.37 |
0.0±0.0 |
2.45±0.37 |
1.87±0.21 |
0.0±0.0 |
1.87±0.21 |
2.42±0.4 |
0.0±0.0 |
2.42±0.4 |
|
10 |
2.32±0.57 |
0.0±0.0 |
2.32±0.57 |
2.31±0.41 |
0.0±0.0 |
2.31±0.41 |
2.31±0.56 |
0.0±0.0 |
2.31±0.56 |
2.02±0.31 |
0.0±0.0 |
2.02±0.31 |
|
5 |
0 |
10.7±0.8a |
0.2±0 |
11.0±1.0a |
23.2±1.5a |
0.4±0.2 |
23.7±1.7a |
41.5±1.5a |
0.9±0.2 |
42.5±1.9a |
23.2±1.3a |
0.2±0.2 |
23.5±1.3a |
2.5 |
8.00±0.4b |
0.2±0.2 |
8.25±0.4b |
19.7±1.2a |
0.5±0.2 |
20.2±1.2a |
29.0±0.8a§ |
0.5±0.2 |
29.5±0.6a§ |
13.0±0.9b§ |
0.2±0.2 |
13.2±0.8b§ |
|
5 |
6.75±0.2cª |
0.2±0.2 |
7.00±0.4c |
19.7±1.5a |
0.5±0.2 |
20.2±1.7a |
23.7±1.1a* |
0.5±0.2 |
24.2±1.2a* |
11.5±0.6b* |
0.0±0.0 |
11.5±0.6b* |
|
7.5 |
5.50±0.2§ |
0.0±0 |
5.50±0.2ª |
18.0±0.9b |
0.2±0.2 |
18.2±0.7b |
21.5±0.6a* |
0.2±0.2 |
21.7±0.8a* |
8.25±0.7c* |
0.0±0.0 |
8.25±0.7c* |
|
10 |
4.75±0.6§ |
0.2±0.2 |
5.00±0.8§ |
13.0±0.7c§ |
0.2±0.2 |
13.2±0.9c§ |
21.5±1.5a* |
0.0±0 |
21.5±1.5a* |
7.75±0.6* |
0.0±0.0 |
7.75±0.6* |
|
10 |
0 |
24.2±1.1a |
0.75±0.2 |
25.0±1.2a |
51.5±1.3a |
1.0±0.2 |
52.5±1.0a |
75.2±2.1a |
1.75±0.4 |
77.0±1.4a |
35.7±1.6a |
0.50±0.2 |
36.2±1.4a |
2.5 |
19.2±0.8aª |
0.25±0.2 |
19.5±0.6aª |
37.7±2.8aª |
1.0±0.2 |
38.7±2.5aª |
61.7±1.2aª |
0.50±0.2 |
62.2±1.2aª |
35.5±2.3a |
0.25±0.2 |
33.2±1.0a |
|
5 |
17.5±0.6a§ |
0.00±0.0 |
17.5±0.6a§ |
34.7±2.3aª |
0.50±0.2 |
35.2±2.0a§ |
54.5±2.0a§ |
0.25±0.2 |
54.7±2.0a§ |
32.5±2.5b |
0.00±0.0 |
30.2±0.4a |
|
7.5 |
16.5±0.6a§ |
0.00±0.0 |
16.5±0.6a§ |
32.7±1.8a§ |
0.00±0.0 |
32.7±1.8a§ |
47.7±2.9a* |
0.00±0.0 |
47.7±2.9a* |
29.5±2.2b |
0.25±0.2 |
27.0±1.3a2 |
|
10 |
12.0±0.4a* |
0.00±0.0 |
12.0±0.4b* |
27.2±2.0b§ |
0.00±0.0 |
27.2±2.0b* |
41.5±1.6a* |
0.00±0.0 |
41.5±1.6a* |
25.0±2.7bª |
0.00±0.0 |
21.2±0.8a* |
|
15 |
0 |
31.7±1.5a |
0.50±0.2 |
32.2±1.7a |
56.5±2.5a |
1.25±0.4 |
57.7±2.8a |
92.5±1.9a |
1.50±0.4 |
94.0±2.3a |
41.2±2.9a |
1.0±0.2 |
42.2±3.0a |
2.5 |
28.2±1.3a |
0.25±0.2 |
28.5±1.1a |
49.7±0.8a |
0.50±0.2 |
50.2±1.0a |
83.5±1.7a |
0.75±0.2 |
84.2±2.1a |
33.0±1.0a |
0.25±0.2 |
35.7±2.4a |
|
5 |
25.7±1.2a |
0.00±0.0 |
25.7±1.2a |
47.2±1.1aª |
0.00±0.0 |
47.2±1.1aª |
79.2±1.4a |
0.25±0.2 |
79.5±1.4a |
30.2±0.4a |
0.00±0.0 |
32.5±2.5b |
|
7.5 |
23.5±1.1a |
0.00±0.0 |
23.5±1.1aª |
41.5±0.6aª |
0.00±0.0 |
41.5±0.6a§ |
71.7±2.9aª |
0.25±0.2 |
72.0±2.7a§ |
27.0±1.3aª |
0.00±0.0 |
29.7±2.1b |
|
10 |
20.7±1.7aª |
0.00±0.0 |
20.7±1.7aª |
35.0±0.7a* |
0.00±0.0 |
35.0±0.7a* |
64.0±4.0a§ |
0.25±0.2 |
64.2±3.9a§ |
21.2±0.8a* |
0.00±0.0 |
25.0±2.7bª |
|
Table 1 Alteration in the doxorubicin-induced micronucleated polychromatic erythrocytes of mice bone marrow treated with various doses of naringin
* and a=P<0.001, ♣ and b=p<0.01, ♠ and c=p<0.05 and no symbol=non-significant
Alphabets, when naringin (NIN) groups compared to doxorubicin (DOX) group
Symbols, when compared to preceding dose of naringin in the same group
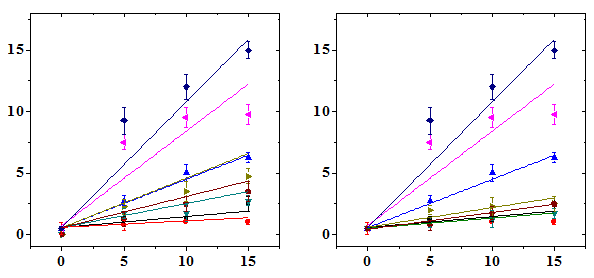
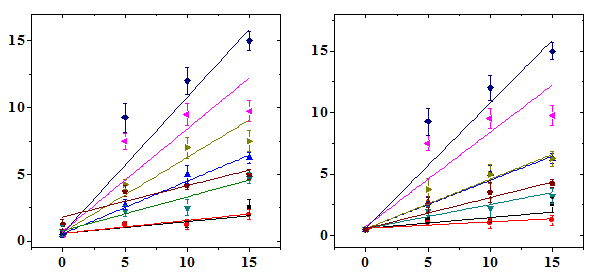
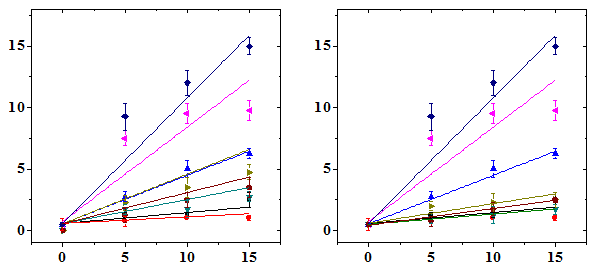
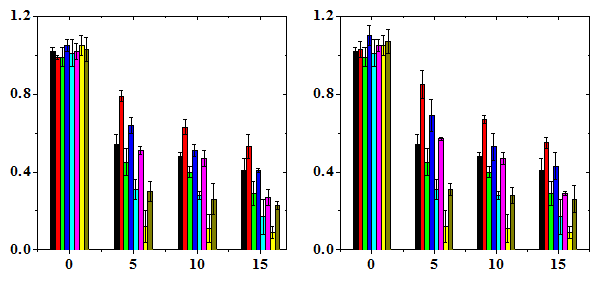
The frequency of micro nucleated normo chromatic erythrocytes (MNCE) is represented separately (Table 2). The NIN treatment alone did not alter the spontaneous frequency of MNCE when compared with the non-drug treated control (Table 2). The mice treated with different doses of DOX showed a dose dependent elevation in MNCE at all the scoring times and a maximum number of MNCE was scored for 15 mg/kg DOX (Figure 2). Similarly, the frequency of MNCE increased steadily with scoring time and a maximum number of MNCE was observed at 72 h post-DOX treatment (Figure 2). The Naringin treatment before DOX administration significantly reduced the frequency of MNCE. However, the pattern of formation of MNCE was similar to that of DOX alone treatment (Table 2). The DOX treatment alone induced two micronuclei in NCE, which were consistently present at 24 to 72 h post treatment, especially at higher doses at 24 h (Table 2). A maximum reduction in the number of MNCE was observed in the animals receiving 10 mg/kg NIN once daily for five consecutive days before treatment with different doses of DOX, where a protection factor of 3.9, 23 and 14.5 was obtained for 5, 10 and 15 mg/kg NIN + DOX treatment (Table 4). The dose response for DOX alone was linear however, for Naringin and DOX linear response was observed only at 24 and 48 h post-DOX treatment (Figure 2). Treatment of mice with different doses of NIN did not significantly alter the spontaneous levels of PCE/NCE ratio that indicates the cell proliferation (Table 3). Administration of mice with different doses of DOX resulted in a dose dependent decline in the PCE/NCE ratio and a highest decline was observed for 15 mg/kg DOX at all post-treatment times (Figure 3). The decline in PCE/NCE ratio was significant when compared with non-DOX treated controls (Table 3). The PCE/NCE ratio continuously declined with scoring time and a maximum decline was observed at 72 h post-DOX-treatment (Figure 1). Treatment of mice with various doses of NIN before DOX-treatment arrested the DOX-induced decline in PCE/NCE ratio in a NIN dose dependent manner as evident by a significant elevation in the PCE/NCE ratio when compared to DOX-treatment alone (Table 3). A maximum rise in PCE/NCE ratio was observed for 10 mg/kg NIN for all doses of DOX at all scoring times (Figure 3).
Treatment |
|
Micronucleated normochromatic erythrocytes (MNCE 1000 ±SEM) |
|
|
|
|
|
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
DOX |
NIN |
12h |
|
|
24h |
|
|
48h |
|
|
72h |
|
|
mg/kg |
mg/kg |
One |
Two |
Total |
One |
Two |
Total |
One |
Two |
Total |
One |
Two |
Total |
0 |
0 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
2.5 |
0.50±0.5 |
0.00±0.0 |
0.50±0.5 |
0.75±0.4 |
0.00±0.0 |
0.75±0.4 |
0.50±0.2 |
0.00±0.0 |
0.50±0.2 |
1.25±0.4 |
0.00±0.0 |
1.25±0.4 |
|
5 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
|
7.5 |
0.50±0.5 |
0.00±0.0 |
0.50±0.5 |
0.00±0.0 |
0.00±0.0 |
0.50±0.1 |
0.00±0.0 |
0.00±0.0 |
0.50±0.2 |
0.00±0.0 |
0.00±0.0 |
0.50±0.1 |
|
10 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
0.50±0.2 |
0.0±0.0 |
0.50±0.2 |
|
5 |
0 |
1.25±0.2 |
0.00±0.0 |
1.25±0.2 |
2.75±0.4 |
0.0±0.0 |
2.75±0.4 |
6.50±0.6b |
1.0±0.0 |
7.50±0.6b |
7.25±1.1b |
2.0±0.0 |
9.25±1.1a |
2.5 |
1.25±0.2 |
0.00±0.0 |
1.25±0.2 |
2.25±0.4 |
0.0±0.0 |
2.25±0.4 |
4.25±0.4 |
0.0±0.0 |
4.25±0.4 |
2.75±0.2§ |
1.0±0.2 |
3.75±0.4* |
|
5 |
1.00±0.2 |
0.00±0.0 |
1.00±0.2 |
2.00±0.4 |
0.0±0.0 |
2.00±0.4 |
3.25±0.8 |
0.5±0.2 |
3.75±0.9 |
2.50±0.8* |
0.0±0.0 |
2.50±0.6* |
|
7.5 |
1.00±0.2 |
0.00±0.0 |
0.75±0.4 |
1.75±0.4 |
0.0±0.0 |
1.75±0.4 |
2.25±0.6ª |
0.0±0.0 |
2.25±0.6ª |
3.50±0.8 |
0.0±0.0 |
1.25±0.2* |
|
10 |
0.50±0.0 |
0.0±0.0 |
0.50±0.1 |
1.00±0.4 |
0.00±0.0 |
1.00±04 |
2.00±0.4§ |
0.00±0.0 |
2.00±0.4§ |
0.75±0.4* |
0.00±0.0 |
0.75±0.4* |
|
10 |
0 |
1.25±0.0 |
0.0±0.0 |
1.25±0.2 |
4.75±0.4a |
0.25±0.2 |
5.00±0.7a |
8.50±0.6b |
1.0±0.2 |
9.50±0.8b |
9.75±1.1a |
2.25±0.2 |
12.0±1.0a |
2.5 |
1.25±0.4 |
0.0±0.0 |
1.25±0.4 |
2.50±0.6 |
0.00±0.0 |
3.50±0.6 |
6.75±0.8b |
0.25±0.2 |
7.00±0.8b |
3.00±0.4ª |
1.2±0.0 |
4.20±0.3ª |
|
5 |
1.00±0.4 |
0.0±0.0 |
1.00±0.4 |
2.25±0.8 |
0.00±0.0 |
2.25±0.8 |
5.00±0.8c |
0.00±0.0 |
5.00±0.8 |
2.00±0.4* |
0.50±0.2 |
3.50±0.8* |
|
7.5 |
1.00±0.0 |
0.0±0.0 |
1.00±0.0 |
1.75±0.7 |
0.00±0.0 |
2±0.7 |
3.50±0.8ª |
0.00±0.0 |
3.50±0.8ª |
1.25±0.2* |
0.00±0.0 |
2.50±0.6* |
|
10 |
1.00±0.0 |
0.00±0.0 |
1.00±0.0 |
1.25±0.4 |
0.00±0.0 |
1.25±0.4 |
2.25±0.8ª |
0.00±0.0 |
2.25±0.8ª |
1.75±0.4* |
0.00±0.0 |
1.75±0.4* |
|
0 |
2.25±0.6 |
0.25±0.2 |
2.50±0.6 |
5.25±0.8a |
0.50±0.2 |
6.25±0.4a |
8.50±0.6b |
1.25±0.2 |
10.75±0.8a |
11.7±0.6a |
3.25±0.5 |
15.0±0.7a |
|
2.5 |
1.75±0.4 |
0.25±0.2 |
2.00±0.4 |
4.25±0.8 |
0.25±0.2 |
4.75±0.4b |
7.00±0.7b |
0.25±0.2 |
7.50±0.8b |
3.50±0.6* |
1.50±0.4 |
5.00±0.4c* |
|
15 |
5 |
1.25±0.6 |
0.00±0.0 |
1.25±0.6 |
3.25±0.6 |
0.00±0.0 |
3.25±0.6ª |
6.00±0.7b |
0.25±0.2 |
6.25±0.6c |
4.25±0.4 |
0.0±0.0 |
4.25±0.4ª |
7.5 |
0.75±0.4 |
0.00±0.0 |
1.00±0.2 |
2.75±0.4 |
0.00±0.0 |
2.75±0.4ª |
4.50±0.6cª |
0.25±0.2 |
4.75±0.6cª |
2.00±0.6* |
1.00±0.4 |
3.50±0.8§ |
|
|
10 |
1.00±0.2 |
0.00±0.0 |
1.00±0.0 |
1.75±0.4 |
0.00±0.0 |
1.75±0.4§ |
2.50±0.6§ |
0.0±0.0 |
2.50±0.6§ |
2.50±0.2ª |
0.00±0.0 |
2.50±0.2§ |
Table 2 Alteration in the doxorubicin-induced micronucleated normochromatic erythrocytes of mice bone marrow treated with different doses of naringin
* and a=P<0.001, ♣ and b=p<0.01, ♠ and c=p<0.05 and no symbol =non-significant
Alphabets, when naringin (NIN) groups compared to doxorubicin (DOX) group
Symbols, when compared to preceding dose of naringin in the same group
Treatment |
|
polychromatic erythrocytes/normochromatic erythrocyte ratio (1000 ±SEM) |
|||
|---|---|---|---|---|---|
DOX |
NIN |
12h |
24h |
48h |
72h |
mg/kg |
mg/kg |
||||
0 |
0 |
1.02±0.02 |
0.99±0.05 |
1.01±0.07 |
1.05±0.05 |
2.5 |
1.02±0.03 |
0.99±0.02 |
1.01±0.02 |
1.02±0.03 |
|
5 |
1.01±.04 |
1.0±0.01 |
1.02±0.05 |
0.99±0.01 |
|
7.5 |
0.99±0.01 |
1.05±0.03 |
1.02±0.04 |
1.03±0.06 |
|
10 |
1.03±.04 |
1.10±0.05 |
1.05±.03 |
1.07±0.06 |
|
5 |
0 |
0.54±0.05* |
0.45±0.07* |
0.31±0.05* |
0.12±0.08* |
2.5 |
0.61±0.04* |
0.53±0.04* |
0.45±0.07* |
0.24±0.04* |
|
5 |
0.76±0.06§c |
0.62±0.8*a |
0.47±0.08* |
0.26±0.09* |
|
7.5 |
0.79±0.03* |
0.64±0.04* |
0.51±0.02* |
0.30±0.05* |
|
10 |
0.85±0.07 |
0.69±0.08§ |
0.57±0.01* |
0.31±0.03* |
|
10 |
0 |
0.48±0.02* |
0.4±0.03* |
0.28±0.02* |
0.11±0.07* |
2.5 |
0.5±0.03* |
0.46±0.04* |
0.42±0.05*c |
0.21±0.08* |
|
5 |
0.58±0.04* |
0.49±0.05* |
0.45±0.08* |
0.24±0.07* |
|
7.5 |
0.63±0.06* |
0.51±0.03*c |
0.47±0.04*b |
0.26±0.08* |
|
10 |
0.67±0.02*b |
0.53±0.07* |
0.49±0.03*a |
0.28±0.04* |
|
15 |
0 |
0.41±0.06* |
0.29±0.06* |
0.17±0.09* |
0.09±0.03* |
2.5 |
0.46±0.05* |
0.36±0.04* |
0.22±0.05* |
0.16±0.04* |
|
5 |
0.48±0.06* |
0.39±0.07* |
0.26±0.08* |
0.2±0.06* |
|
7.5 |
0.53±0.06* |
0.41±0.01* |
0.27±0.04* |
0.23±0.02*b |
|
10 |
0.55±0.03* |
0.43±0.07* |
0.29±0.01* |
0.26±0.07* |
|
Table 3 Alteration in the doxorubicin-induced cell proliferation (polychromatic erythrocytes to normochromatic erythrocyte ratio) in the mice bone marrow treated with various doses of naringin
* and a=P<0.001, ♣ and b=p<0.01, ♠ and c=p<0.05 and no symbol = non significant
Alphabets, when naringin (NIN) groups compared to doxorubicin (DOX) group
Symbols, when compared to preceding dose of naringin in the same group
Dox |
Naringin |
Protection Factor (PF) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
MPCE |
MNCE |
|||||||||||||||||
12h |
24h |
48h |
72h |
12h |
24h |
48h |
72h |
|||||||||||
One |
Total |
One |
Total |
One |
Total |
One |
Total |
One |
Total |
One |
Total |
One |
Total |
One |
Total |
|||
5 |
2.5 |
1.36 |
1.36 |
1.14 |
1.13 |
1.36 |
1.36 |
1.14 |
1.13 |
1.00 |
1.00 |
1.28 |
2.25 |
1.28 |
1.38 |
2.70 |
3.24 |
|
5 |
1.72 |
1.70 |
1.14 |
1.13 |
1.72 |
1.70 |
1.14 |
1.13 |
1.50 |
1.50 |
1.50 |
2.57 |
1.77 |
2.00 |
1.80 |
2.33 |
||
7.5 |
2.34 |
2.42 |
1.26 |
1.27 |
2.34 |
2.42 |
1.26 |
1.27 |
1.50 |
1.50 |
1.80 |
3.60 |
2.66 |
3.00 |
2.25 |
2.91 |
||
10 |
2.98 |
2.83 |
1.83 |
1.84 |
2.98 |
2.83 |
1.83 |
1.84 |
1.00 |
1.00 |
2.25 |
6.00 |
4.57 |
5.14 |
3.00 |
3.88 |
||
10 |
2.5 |
1.26 |
1.28 |
1.35 |
1.34 |
1.26 |
1.28 |
1.35 |
1.34 |
1.00 |
1.00 |
2.12 |
1.28 |
1.60 |
1.86 |
4.11 |
3.53 |
|
5 |
1.40 |
1.45 |
1.48 |
1.49 |
1.40 |
1.45 |
1.48 |
1.49 |
1.50 |
1.50 |
2.42 |
1.50 |
2.18 |
2.15 |
6.16 |
5.75 |
||
7.5 |
1.49 |
1.55 |
1.57 |
1.61 |
1.49 |
1.55 |
1.57 |
1.61 |
1.50 |
1.50 |
3.40 |
1.80 |
3.42 |
4.00 |
12.30 |
15.33 |
||
10 |
2.20 |
2.25 |
1.92 |
1.96 |
2.17 |
2.25 |
1.92 |
1.96 |
1.00 |
1.00 |
5.66 |
2.25 |
3.00 |
3.50 |
18.5 |
23.00 |
||
15 |
2.5 |
1.11 |
1.12 |
1.12 |
1.13 |
1.11 |
1.12 |
1.12 |
1.13 |
1.40 |
1.33 |
1.26 |
1.35 |
1.23 |
1.32 |
3.73 |
3.22 |
|
5 |
1.23 |
1.25 |
1.18 |
1.21 |
1.23 |
1.25 |
1.18 |
1.21 |
2.33 |
2.66 |
1.72 |
2.09 |
1.45 |
1.60 |
5.60 |
4.83 |
||
7.5 |
1.36 |
1.38 |
1.35 |
1.38 |
1.36 |
1.38 |
1.35 |
1.38 |
7.00 |
8.00 |
2.11 |
2.55 |
2.00 |
2.17 |
7.46 |
7.25 |
||
10 |
1.60 |
1.60 |
1.62 |
1.65 |
1.60 |
1.60 |
1.62 |
1.65 |
7.00 |
8.00 |
3.16 |
3.83 |
3.55 |
4.11 |
11.20 |
14.50 |
||
Table 4 Protection factor for the micronucleated erythrocytes of mice treated with different doses of doxorubicin hydrochloride after naringin treatment.
Micronucleated Normochromatic Erythrocytes/1000
Doxorubicin (mg/kg b. wt.)
Polychromatic/Normochromatic Erythrocyte Ratio
Doxorubicin (mg/kg b. wt.)
Despite extensive research, the mechanism underlying the genotoxicity induced by DOX is still not well understood. One of the leading hypotheses suggests that DOX increases free-radical production and subsequently damage cell DNA.42‒44 DOX is known to intercalate into cellular DNA causing topological changes in the DNA molecule.45,46 Moreover, DOX is also known to inhibit the action of topoisomerase II, which is essential during DNA replication. All these actions of DOX change the fidelity of DNA leading to cytotoxicity. The micronucleus assay is a simple technique to assess DNA lesions in the eukaryotic cells. The micronuclei are acentric fragments or a complete chromosome that fail to attach to the mitotic spindle during cytokinesis and hence get excluded from the main cell nucleus. Micronuclei are one of the generally studied cytogenetic markers, which illustrate early biological effects related to DNA-damaging agents despite the fact that various agents may employ different mechanisms to produce micronuclei, including chromosome breakage (clastogenesis) and spindle disruption (aneugenesis).46Therefore, effect of Naringin was examined on the genotoxic effect of doxorubicin in mice bone marrow cells by micronucleus assay.
The clastogenic effect of DOX in rodent bone marrow cells is well documented.47‒49 The rise in micronuclei induction by DOX treatment has been reported in mice bone marrow earlier.13‒16 A similar effect has been observed in the present study where DOX treatment elevated the frequency of MPCE and MNCE in a dose dependent manner at all post-DOX treatment times. The report regarding the reduction in the DOX-induced MN by NIN are unavailable this is probably the first report, where NIN pre-treatment has been found to abate the DOX-induced micronuclei in a NIN dose dependent manner. NIN has been reported to reduce the radiation and bleomycin-induced micronuclei and molecular DNA damage in mice bone marrow and cultured V79 cells.13,3,6 Similarly, NIN has been found to reduce cytosine arabinoside (Ara C)-induced micronuclei49 and protect the cells against H2O2-induced oxidative DNA damage and apoptosis.46 The extract of Aegle marmelos has been reported to reduce the DOX-induced micronuclei in bone marrow cells of mice and V79 cells.36,37 Similarly, rosmarinic acid, a phytochemical has been reported to protect against the doxorubicin-induced DNA damage and micronuclei in V79 cells.29 Other chemicals like captopril and desferrioxamine have also been reported to reduce DOX-induced micronuclei in mice.17‒19 Vitamin A and vitamin C have been reported to reduce the incidence of DOX-induced chromosomal damage earlier. The frequency of MPCE declined at 72h whereas MNCE increased substantially in the DOX and NIN+DOX treated groups. The decline in MPCE is due to the removal of highly damaged cells and supply of new erythrocytes, whereas highest elevation in MNCE at 72 is due to the maturation of MPCE into MNCE that takes approximately 24h (Table 1) (Table 2). A similar effect has been observed earlier.13‒33 Naringin has been reported to protect against the bleomycin-induced micronuclei in cultured human lymphocytes.11
DOX caused a dose dependent decline in the PCE/NCE ratio indicating its ability to inhibit cell proliferation. An identical effect has been observed earlier with DOX and other topoisomerase II inhibitor VM-26.17,18 NIN pre-treatment arrested the DOX-induced decline in PCE/NCE ratio, and the greatest effect was observed for 10mg/kg NIN. A similar effect has been reported earlier, where NIN has been found to inhibit radiation-induced decline in the PCE/NCE ratio and cell proliferation in V79 cells. Likewise, Aegle marmelos extract has been reported to reduce the DOX-induced decline in the PCE/NCE ratio in mice bone marrow. Captopril has also been reported to inhibit DOX-induced PCE/NCE ratio in mice bone marrow. The DOX may have utilized multiple pathways to inflict damage to cellular DNA. DOX is generally believed to induce the formation of oxygen free radicals including superoxide free radicals.7‒9 Superoxide anion radicals generated by DOX react with hydrogen peroxide and form highly reactive hydroxyl radicals via the iron catalyzed Haber-Weiss reaction. The secondarily derived hydroxyl radicals are highly reactive and can cause protein and DNA damage.8,9 The presence of iron facilitates generation of DOX-induced oxygen free radicals by the formation of DOX-iron complexes.6‒9 The oxygen radicals produced by DOX may interact with cellular DNA to generate DNA adducts, DNA strand breaks and alkali labile sites. These DNA lesions induced by DOX may be subsequently converted into double strand breaks and finally into micronuclei after a cell decides to divide. Our recent study has reported the induction of DNA adducts by DOX in mice.12 Apart from free radical-induced DNA breakage, DOX has been reported to intercalate into DNA and inhibit the action of enzyme topoisomerase-II that plays a crucial role in the segregation of newly replicated parts of intertwined, condensation and decondensation of chromosomes and super coiling of intracellular DNA.2‒9 DNA topoisomerase-II also catalyzes the breakage and reunion of both DNA strands, relax superhelical twist, catenates or decatenates circular DNA. DNA topoisomerase-II performs these topological transformations by transporting one double stranded DNA segment through an enzyme-mediated transient double strand break into another. Several DNA strands are rejoined after completion of the above process.16‒21 DOX being a potent DNA topoisomerase-II inhibitor stabilizes DNA double strand breaks and does not allow them to rejoin leading to cell death.22‒24 These DNA double strand breaks may be converted into chromosomal breaks during strand break repair32 and subsequently end up as micronuclei once the cell divides. The doxorubicin intercalation into DNA has been reported to cause its torsion that result in the neucleosome destabilization, which may have also contributed to the formation of micronuclei.
The exact mechanism of reduction in the DOX-induced DNA damage in the form of micronuclei by Naringin is not clearly understood. Naringin may have employed multiple putative pathways to alleviate DOX-induced micronuclei formation. Naringin may have neutralized the DOX-induced free radicals and reduced the damage to cellular DNA. In our earlier study Naringin has been reported to scavenge O2·- and ·OH radicals in a dose dependent manner.3 Presence of NIN before DOX treatment may have also inhibited the formation of DOX-iron complexes by occupying the free coordination sites of iron and thus reducing the generation of secondary ·OH radicals. Naringin has been reported to protect against the iron-induced oxidative stress and occupy free iron coordination sites.11,13 Up regulation in antioxidant status by Naringin may have mitigated DOX-induced ROS formation reducing the genomic damage. Naringin has been reported to increase the antioxidant status in vivo. Naringin may have also suppressed the formation of DOX-induced DNA adduct and reduced the micronuclei formation. The Naringin has been reported to reduce the formation of DNA adduct earlier. The presence of Naringin may have also inhibited the action of DOX on topoisomerase-II enzymes and thus reduced the DNA damage. Although no attempt has been made to investigate molecular mechanism of action of Naringin, there is no reason to believe that Naringin may have not utilized this pathway for exerting its action. DOX has been reported to induce transactivation NF-kB and COX-II15‒19 and presence or Naringin may have blocked NF-kB activation reducing DOX-induced DNA damage and cell death in mice bone marrow. Naringin has been reported to suppress the activation of NF-kB and COX-II,46‒47 which may have also reduced DOX-induced micronuclei and cell death in the present investigation. Naringin may have transactivated Nrf2 signalling pathway, which would have raised the antioxidant status of DOX treated cells causing reduced formation of micronuclei. Naringin has been found to elevate Nrf2 signalling recently.49 Naringin pretreatment reduced the DOX-induced micronuclei formation and raised PCE/NCE ratio. Naringin may have reduced DNA damage by neutralizing the DOX-induced free radicals, increasing the antioxidant status and suppression of DOX-induced DNA adducts formation. Apart from this Naringin may have employed molecular pathways to alleviate DOX-induced DNA damage where it may have restored the topoisomerase-II activity, blocked the transactivation of NF-kB, repressed the cyclooxygenase-II and activated Nrf2 pathway. Naringin, a grape fruit flavanone may be used to reduce the DOX-induced toxicity in clinical situations.
The authors are thankful to the Indian council of Medical Research, Govt. of India, New Delhi, India for financial support vide grant No. 45/16/2002/PHA/BMS dated July 28th, 2003 to carry out this study.
Author declares that there is no conflict of interest.

©2016 Jagetia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.