International Journal of
eISSN: 2573-2889


Review Article Volume 1 Issue 1
1Department of Biochemistry and Biotechnology, University of Gujrat, Pakistan
2Department of Biotechnology, University of Gujrat, (Sialkot sub-campus), Pakistan
3Faculty of sciences Quaid-i-Azam University, Pakistan
4Department of Bio Sciences, CIIT, Pakistan
Correspondence: Muhammad Naveed, Department of Biochemistry and Biotechnology, University of Gujarat, Pakistan, Tel +92 301 5524624
Received: December 12, 2016 | Published: December 28, 2016
Citation: Naveed M, Tariq K, Sadia H, et al. The life history of pyrroloquinoline quinone (PQQ): a versatile molecule with novel impacts on living systems. Int J Mol Biol Open Access. 2016;1(1):29-46. DOI: 10.15406/ijmboa.2016.01.00005
Pyrroloquinoline quinone (PQQ) acting as redox cofactor of Glucose dehydrogenase is orthocyclic antioxidant acting under multifarious environmental stress and rich conditions in prokaryotes as well as eukaryotes. Microbes are the exclusive source of PQQ biosynthesis and for biocatalysis of glucose into gluconic acid and 2-ketogluconic acid by gram positive and negative bacteria. This study focus to describe PQQ biography (1979-2016) highlighted its applications acts as biocatalyst by enhanced production of NADH from pqqC, involved in several important mechanisms like bacterial energy transduction by m-ATPase, production of biocontrol substances, growth stimulating activities and DNA repair. PQQ acting as anti-neurological, anti-degenerative, anti-melanogenic and anti-cancer agent due to its antioxidant nature by scavenging free radicals. PQQ is modulator of immunity by CD4 cells count and IL-2, sleep maintenance by PGC-1 alpha pathway and inflammation due to ROS. The mineral phosphate solubilization results in plant growth promotional, biocontrol, antifungal and ISR activities. PQQ nanoparticles used for production of Biofuels cells and sulfonated polymers. It acts as a modulator of diverse signaling pathways like STAT, MAPK, JAK, JNK, P13K/Akt, mTOR, EGFR and Raps for cell proliferation, differentiation, apoptosis, Box translocation and metabolic pathways by phosphorylation of ADP and suppression of reactive oxygen species. It protects from oxidative stress by acting as Alpha AA modulator of lysine metabolism, TrxR1 in selenium metabolism, low density lipoprotein in lipid metabolism, ATP production in Energy, Glucose and carbohydrate metabolism. It has been structurally characterized with bioinformatics tools and future perspectives being molecular modeling and docking for analytical drug design.
Keywords: life history, pyrroloquinoline quinine, novel biomolecule, living systems, microorganism, plants and animals
Pyrroloquinoline quinone (4, 5-dihydro(4), 5-dioxo(1)H-pyrroloquinoline-2,7, 9 tricarboxylic acid) being a three ortho cyclic quinone substitutes the role of a redox-cofactor for various bacterial dehydrogenases such as methanol, ethanol and glucose dehydrogenases1‒6 also termed as methotaxin.7 PQQ structure prediction shows that it has been originated from tyrosine and Glutamic-acid8 but the pathway for PQQ biosynthesis is whist in process of identification.9 PQQ being involved in Gluconic acid production and biosynthesis by microbes. GDH-A and GDH-B (s-GDH) are soluble enzymes.10 PQQ synthesizing operon containing 6-7 genes i.e. (pqqABCDEF/G)11,12 and orientation effects13 and biocatalysis are carried by bioelectric oxidation of glucose into gluconic acid.14 It acts as potent microbial growth stimulant and diversified role in antibiotics as chief biological control determinant for plant pathogens.15 PQQ-GDH has role in direct electron transfer of bacterial energy transduction and ATP synthesis during oxidation.16,17 The soluble membrane bound serine and threonine kinases repair damage of DNA by radiation.18 PQQ has diverse roles in animals like NMDA mediated receptor in neurological injury, repair and improved memory by ERK1/2 pathway pathway.19 PQQ is a powerful anti-melanogenic agent and quite effective against disorders related to hyper pigmentation.20 It plays role in MAPk kinase activation and tyrosyl phosphorylation of ERK2 by production of CD4+ T lymphocytes.21 PQQ promotes and improves neonatal development and reproduction involving mitochondrial biogenesis and cell signaling pathways.22 PQQ plays vital role in liver fibrogensis23 and in signal transduction via mitochondrial biogenesis.24 It has diverse functions in insulin resistance and aging by creation of new mitochondria.25
In plants PQQ has phosphate solubilizing activities, plant growth promotion, antifungal activities, induces systematic resistance and symbiosis by acting as an antioxidant.26‒28 In modern era of technology PQQ has been extensively used in bio-electrocatalysis, nanotechnology and polymer based technologies.29‒31 In this review we have focused upon bioinformatics based structural analysis of PQQ-GDH having propeller' fold superbarrel made up of 8-sheet `propeller blades' with tryptophan docking motifs.32 Five transmembrane segments in N-terminal region while C-terminal region having a huge binding domain for PQQ conserved with functions related to catalytic center.30 The oxidation of D-glucose to D-gluconate by m-GDH is catalyzed by Pseudomonas & Gluconobacter.33 PQQ on c-terminal being conserved in Alcohol dehydrogenase of Pseudomonas putida have same ontology.30 There are ciprocityin acetic acid bacteria for ethanol resistance PQQ-ADH.34 The mGDH appears to be unique in mechanism of PQQ-binding with bivalent metal ions by steady continuous production of gluconolactone.35 The mechanism of docking involves activator ammonia with its unique cytochromes.36
The S-GDh has catalytic potential, adduct-forming ability, reversible substrate binding, direct transfer of a H− and oxidation of PQQH2 by an electron acceptor.37 It makes headway via undivulged pathway that requiring six genes, pqqC to –F, pqqC as rate controlling gene38 and supply energy for growth on alcohol or aldoses substrates.39The biochemical pathways, physiological, cellular and molecular processes involving PQQ are linked to metabolic pathways for protection against oxidative stress by scavenging Reactive oxygen species(ROS).
Sources of PQQ
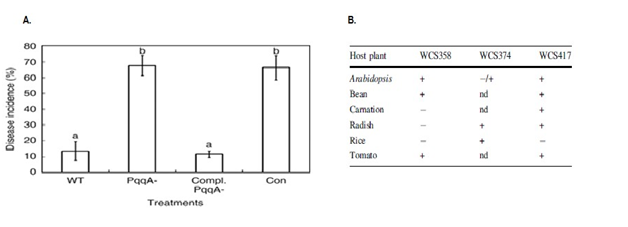
PQQ as catalytic center of gram negative bacteria,40 discovered in 1979 acting as redox agent.41 Many bacteria comprehend the genes required for PQQ biosynthesis like Klebsiella pneumonia and Acinetobacter calcoaceticus, but Salmonella typhimurium and Escherichia coli are not able to synthesize PQQ (Figure 1), because they lack genes encoding them.42,43 The obtained nucleotide and protein sequence from Escherichia adecarboxylata, E. adecarboxylata, reclassified as Leclercia adecarboxylatahomology of 99% of E. Cloacae subsp. with Enterobacteriaceae by extrication of glucose dehydrogenase.30 GDH-A has been reported in numerous bacterial species like Klebsiella aerogenes, Escherichia coli P. Ae ruginosa, G. Suboxydans, Acinetobacter calcoaceticus, and A. Lwoffi while s-GDH found in A. Calcoaceticus. PQQ is scarcely present animal and plant tissues, could not be produced by plants and animals. Produced in plant-associated systems by rhizobacterial source.44 PQQ is important growth cofactor the techniques and methodologies should be more clearly defined for extraction of its bioactive form.
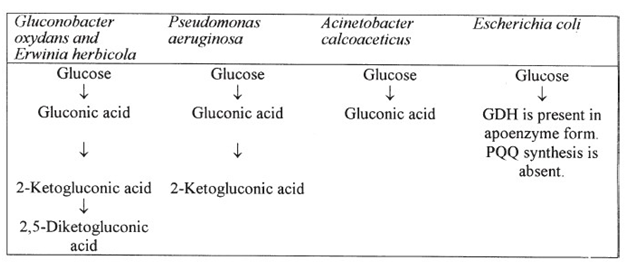
PQQ biosynthesis
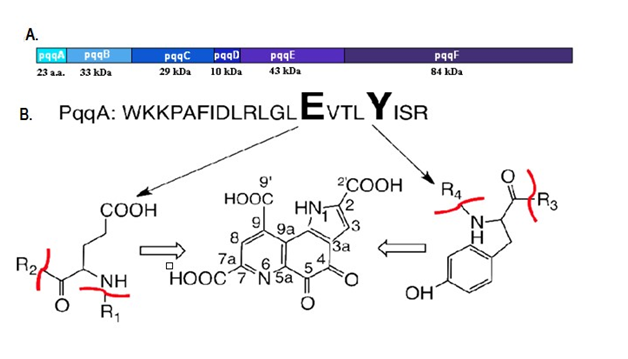
The K. Pneumonia is an excellent host microorganism for PQQGDH-B production(Kojima et al.2000).The gene pqqA in bacteria conceal 23-29 amino acids, pqqC being highly conserved in K. Pneumoniae 23 amino acids (Figure 2),45,46 PqqA is 20 folds larger than the PqqC or PqqE.47 The aldehydes, ketones and organic acids are excreted by gluconobacter sp, being catalyzed by dehydrogenases in periplasmic space.48 The pqqC gene with 29 kDa molecular weight (250 residues) acts as catalystfor synthesis of PQQ49 (Figure 3) compressed into hydrophobic helix bundle49 of 90 residues in pqqD.50 The pqqDGCBA in Methyl bacterium strain being distinguished by complementation resolution, pqq-FAB of K. Pneumoniaesequence analysis51 showed 11 genes (A-B-C-D-E-F-H-I-J-K and M) present in PQQ-operon of P. Fluorescens were recognized.52 The PQQ operon of G. Oxydans contains pqqABCDEF53,54 and pqqABCDEF in 621H gene of G. Oxydans.55 The structural characterization shows that PqqC is important for catalyzing the reaction, PqqD for production of PQQ, PqqD for interaction and PqqE for cluster formation while a functional characterization is required for PQQ biosynthetic process to be involved in various biological processes.
PQQ as biocatalyst
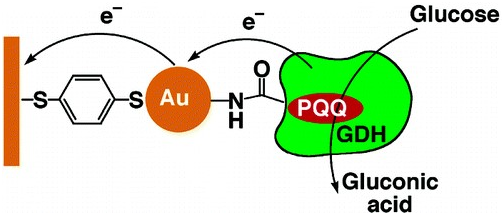
PQQ acts as a potent biocatalyst the fact is proved by the reaction rate at 11,800 presuming biologically active structure due to oxidation of glucose in bioelectrocatalytic way (Figure 4).56 (PQQ)-dependent (GDH) acting as electron sink57 and NADPH enhancing the production of PQQ by PqqC-D, product inhibition.58 The endogenous PQQ genes in K. Pneumoniae along with heterologous expression in Escherichia coli were over expressed in T7 promoter.59 PQQ being enzymatic biocatalyst is involved in many-oxidation reduction processes can be used for production of many biocatalytic products of industrial significance.
PQQ as microbial growth stimulant
PQQ has growth stimulating effect by decrease of the lag period and subsequently increasing growth speed, yield and induction of cell reproduction60 by subsequent growth increase at the exponential phase acting assecondary type of growth stimulant (Figure 5).61,62 PQQ acts as a growth factor for many bacteria under stress and normal conditions63 and also reported for growth stimulating activities by formation of growth stimulating factors, apoqunioproteins by free PQQ and adduct.64 The biologically active PQQ found in periplasmic spaces is known to be growth stimulant and isolation of cofactor containing active site can lead to elevated production of PQQ.
PQQ role in antibiotics
The antibiotics phenazine, 1-carboxylic acid and 2,4-diacetylphloroglucinol are used as bio-control determinants of Pseudomonas spp65 providing unequivocal evidence that antibiotics have a role in the suppression of disease.66 Six pqq genes and one GDh gene has been identified in R. Aquatilis strains, found necessary for biosynthesis of the Antibacterial substqnceABS, as biocontrol of crown gall disease.67 The (GDHm) is a 86 kDa68 double mutants of PQQ are more sensitive and ndvB stimulates antibiotic resistance by sequestration of drugs in cyclic glucans and ethanol oxidation genes activates antibiotic resistance (Figure 6).69 PQQ can suppress damping-off caused by the oomycete by genes (sup 5&6) for biocontrol activities (Table 1),70 An associated cytochrome c approaching the PQQ for direct electron transfer.71 The Pseudomonas putida’s being chloramphenicol resistant bacterium is involved in metabolism, cellular regulation and stress with gene regulation by efflux transporters and biosynthesis of proteins.72 The physiological roles played by PQQ for regulation of intracellular polyamines and other perspective should be genetically worked out for production of antibiotics.
Gene |
Size |
Function |
Reference |
PqqEM. extorquens |
384 |
Biofilm-specific antibiotic resistance, ΔndvB, biocontrol activities at logarithmic phase |
47, 48 |
PqqBM. extorquens |
299 |
Same biocontrol and antibiotic resistance functions like PQQE |
45, 68 |
PqqCA. calcoaceticu |
252 |
Biofilm,antibioticresistance,biocontrol like PQQB |
54, 67,42 |
PqqD, R. aquatilis |
85 |
Requires NADH , O2, biocontrol activities at logarithmic phase |
29 |
pqqF Klebsiella pneumoniae |
83,616 Da |
Antibiotics pyoluteorin (Plt) and 2,4-diacetylphloroglucinol (Phl) production for disease suppression |
26,28 |
Table 1 Antibiotic and biocontrol activities of PQQ operon
PQQ in bacterial energy transduction
PQQ-GDH from A. Calcoaceticus and glucose oxidase from A. Nigar with cytochrome b(562) as electron acceptor in transfer of oxidoreductases,the quaternary structure of which do not have transfer subunit for electrons (Figure 7).73 Biologically active energy being conserved NADH2oxidations during the periplasmic oxidation of PQQH2.74 Two forms of metabolic energy can be inter-connected by the action of ion-translocating ATPases.75 PQQ is involved for modification and oxidation by cell signaling acting as a Redox cofactor and powerful antioxidant.76,77 A pronounced insight should be made on pathways related energy production due to attribution of PQQ on mitochondrial functions and ATPase production.
ATP synthesis during oxidation
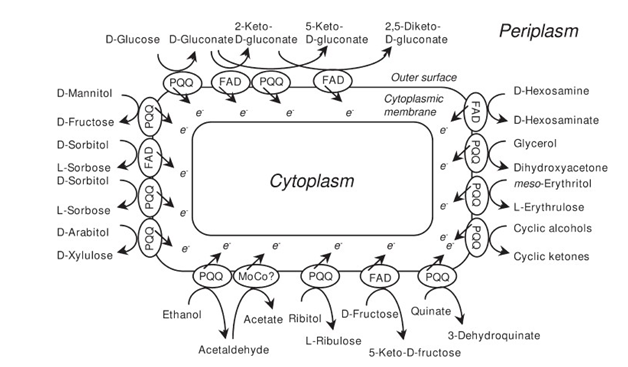
PQQ/PQQH2 oxidation-reduction involved in transfer of electrons to electron acceptors by reckon on the specific quinoprotein enzyme, cytochrome c (Cu-protein), NADH dehydrogenase and cytochrome b.78 Glucose being tremendous energy generation substance for transport of secondary solutes in PQQ-GDH a powerful role in energy metabolism(Figure 8)(Figure 9).79 PQQ-dependent production of gluconic acid by Acinetobacter, Agrobacterium and Rhizobium species. PQQ enhances energy production (ATP) by protecting existing colossal mitochondrial biogenesis and high number of m-dehydrogenases (Figure 10).80 2PQQ catalyzes the oxidation of thiol groups perilously allied with the function of two proteins, i.e. thioredoxin and phosphoribulose kinase in catalysis and stabilization of protein structure.81
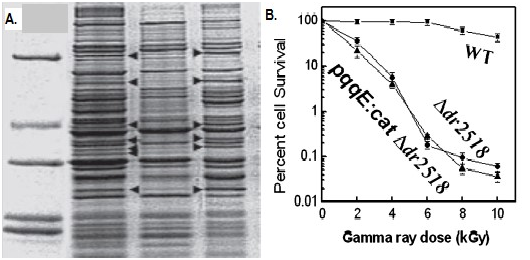
PQQ intracellular signaling in DNA repair
Oxidation of lipids, proteins, and nucleic acids hinder membrane function and integrity, inactivation of enzymes, modification of lipoproteins, and chemical alteration of DNA.82 PQQ for m-bound soluble kinases like serine-threonine involved in repair of radiation induced DNA damage, repair and recombination by strong interaction of yfgL mutant wihaving wild recA genes through a cell signaling (Figure 11).83 In DNA end resection requirement for CDK1, DNA damage checkpoint activation for homologous recombination provides evidence for PQQ for induction process in repair of DNA by kinase protein as radiation resistant substance.84 PQQ synthesis manifests sensitivity to gamma rays, double break in DNA helix, repair of STK domain (eukaryotes and prokaryote).85 The pqqD is essential for PQQ biosynthesis in K. Pneumonia.86 PQQ-dependent sugar dehydrogenase gene with auxiliary activities and structural hallmarks provides distinctive penetration into the mechanism of oxidation by metabolism pathway of sugars.87 PQQ with its metal ions, NADH mediated functions and transcription factors can lead to repair of DNA cleavage sites.
PQQ in neurological injury and repair
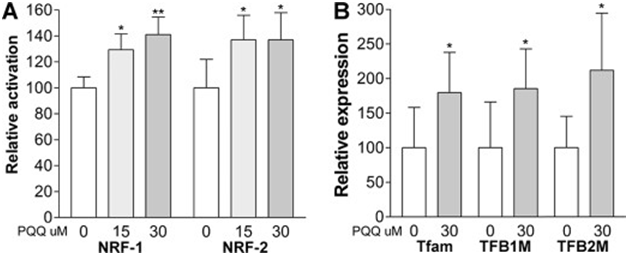
The compilation of oxygen-imitative free radicals can oxidize the NMDA receptor acquired the degree of neuroprotection, inflammation and neurotoxicity due to excessive glutamate.88,89 Pyrroloquinoline quinone is an enhancer of nerve growth factor NGF production in vitro,90 not able to damage barrier between blood and brain enhancing activation of first NRF-1and second nuclear respiratory factor (NRF-2).91,92 PQQ inhibiting cytotoxicity of the alpha-synuclein variants and amyloid fibril formation and believed to be a strong candidate as a compound for treatment of PD (Figure 10),93 Pyrroloquinoline quinone triggers ERK1/2 pathway, regulation of glutathione, modulation of Bcl-2 and Bax.94 PQQ impoverish pain sensation by protection against chronic neuropathy, sciatic nerve irritability and injury in animals was reduced with PQQ and enhanced sequential oxidative +3 stress by restoration of neurotransmitter levels in brain.95 PQQ acting as anti-neurodegenerative compound from glutamate damage96,97 by reversible action of middle artery occlusion98 affecting learning ability and memory function of rats model significantly.99 The disorders like Alzheimer's, dementia and Parkinson's disease, stroke, Huntington’s disease are neurodegenerative.100‒102 PQQ prevents accumulation of alpha-synuclein and amyloid beta proteins.103 PQQ provides advanced level of protection on endothelial cells of mouse brain from Gluco-damage by suppression of ROS and cell death by blocking signaling pathway of JNK.104‒107 The treatment of proliferated Schwann cells with various concentrations of PQQ enhanced the expression of CREB, c-fos, c-jun and PCNA.108 PQQ on traumatic brain injury (TBI) has found to be neuroprotective109 as measurements of physiological parameters indicated that PQQ restrains function of brain in older people related attention, working and memory.110 PQQ can act as a strong candidate to be used in pahrmacogenomics of brain injuries and other complications.
PQQ role in skin
PQQ is anti-melanogenic agent as melanin is the consequential determinant of diverse hyper-pigmentation disorders, synthesized by various transcriptional factors, tyrosinase-related protein (TRP-1 and -2), and tyrosinase.111 Novel allyl PQQ combination with chlorogenic acid and methyl gentisate is found effective for hyper pigmentation acknowledged at diminution of skin hyper pigmentation (Gold; jhskincareclinic.co.uk).Loss of PQQ causes a reduction in connective tissue growth and repair proved by Cellular studies on fibroblasts leading to friable skin, loss of elasticity and connective tissue health also used in make-up and creams for aging and skin care etc. PQQ contributes on epidermal water dissipation, skin moisture, texture, viscoelasticity, and reduction in mast cell quantity in the dermis and epidermis and quantity of CD3⁺ T-cells giving improved skin barrier and function (Table 2).112 PQQ causing biological aging induced by ultraviolet A UVA in dermal fibroblasts of humans HDFs via ant apoptotic SIRT1- SIRT6-HO‑1 and Nrf2 signaling pathways.113 It protects against long wavelength UVA rays targetingretinoid and alpha hydroxy skin cells as an ingredient in most anti-aging creams. PQQ works on mitochondria involved in cell signaling, cycling, differentiation, and growth increase cellular turnover through exfoliation.114 The American Academy of Anti-Aging Medicine has published a book where PQQ has been enlisted as an ingredient for anti-aging because it preserves mitochondira, slows down hardening of arteries, replaces hormones with bioidentical one’s.115 Due to involvement of PQQ in lysine metabolism and pronounced effects on skin layers it can be a novel component in contribution with other skin compounds in health care industry.
Item |
Group |
Week 0 |
Week 4 |
Week 8 |
Wrinkles |
Placebo |
-0.9±0.7 |
-0.1±0.2 |
-0.5±0.5 |
PQQ |
-1.6±0.5 |
-0.5±0.6** |
-0.6±0.7* |
|
Pigmentation |
Placebo |
-2.3±0.6 |
-1.4±0.4 |
-0.5±0.6 |
PQQ |
-3.6±0.5 |
-1.3±0.6* |
-0.7±0.6** |
Table 2 Effect of PQQ intake on the subjective recognition on of facial skin conditions22
PQQ role in immunity
PQQ regulates MAPk kinase activation and tyrosyl phosphorylation of ERK2 by production of CD4+T lymphocytes116 having immune response by interleukin-2, and growth factors reduced when T-cell proliferation occurs.117 When PQQ supplemented orally in nano-amounts alleviates the mitogens of B and T cells.118 PQQ treatment enhances IgA level, restores mass of GALT119 and induce immunity against bacterial or viral invasion,120 subsequent suppression by increased levels of proteins induced by IFN-β via iNOS, JAK1 and STAT1 signaling pathways (Table 7). PQQ was involved in phosphorylation of 1KKβ, p38 and nF-kB as a pre-inflammatory retort of macrophages121 increase the number of lymphocytes and CD8+cells (Table 3).19 Robust research should be conducted on clinical trials to estimate IL-6 and T lymphocytes to act as immune-suppressive agent.
|
Group |
ΑβTCR+ |
γδTCR+ |
CD4+ |
CD8+ |
PP Lymphocytes |
IG-STD-PN |
2.07 ±0.21 |
0.10 ±0.02 |
1.68 ±0.17 |
0.49 ±0.08 |
IG-PQQ-PN |
2.76 ±0.27 |
0.12 ±0.01 |
2.37 ±0.24 |
0.47 ±0.04 |
|
IE Lymphocytes |
IG-STD-PN |
1.24 ±0.41 |
0.24 ±0.05 |
0.58 ±0.23 |
0.94 ±0.02 |
IG-PQQ-PN |
0.75 ±0.13 |
0.22 ±0.06 |
0.36 ±0.10 |
0.65 ±0.12 |
|
LP Lyphocytes |
IG-STD-PN |
1.12 ±0.20 |
0.28 ±0.06 |
0.53 ±0.07 |
1.05 ±0.22 |
IG-PQQ-PN |
0.83 ±0.11 |
0.27 ±0.05 |
0.43 ±0.05 |
0.72 ±0.10 |
Table 3 Absolute lymphocyte numbers with added PQQ)120
PQQ link to oxidative stress, tolerance and sleep
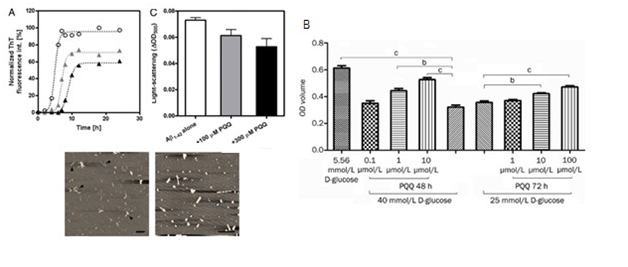
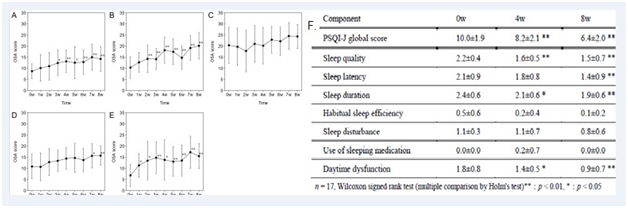
PQQ administration results in considerable improvements in span of sleep, improvements in total duration of sleep, avoid of awakenings at night but not for nightmares (Figure 11),122 being treated with 20mg PQQ for 8 weeks in 17persons.123 The insomnia might be attributed to fatigue and stress which are indirectly involved with oxidative stress and ROS. Oral administration of PQQ, zinc, vitamin E and coenzyme Q10 improves sleep quality and time period.124 Multiple studies should be carried out on persons with impaired sleep to show its correlation with normal sleep cycle.
PQQ role in Inflammation and disease by free radicals
By PQQ optimization a marked reduction in quantitative degree C-reactive plasma protein and Il-6 various urietic markers of oxidative stress endowed consistent with mitochondria-related boosted functions.125 High levels of reactive oxygen species ROS is associated with cellular and mitochondrial damage causing inflammation leading to deteriorative disease126 as potential reformat to oxidative damage in PTU-induced mice kidney.127 Divergent circumstances related to inflammation, oxidative stress, and metabolic dysregulation by increasing mitochondrial biogenesis, enhanced inflammation, and to alleviate the level of endogenous enzymatic and non-enzymatic antioxidants in a subject (Table 4).31 PQQ in osteoarthritis OA can be investigated by the iNOS level execution of novel pharmacological and clinical prevention in the near future.21 In rheumatoid arthritis RA of anti-inflammatory effects of PQQ were interrogated in interleukin (Il)-1β MAP and JNK kinase pathways, hindered by PQQ in IL-1β of Sw982 cells , can be a promising therapeutic agent(Table 4).34 It critically constrains the creation of PGE2 and NO with suppression of COX-2, MIP-1a ,TNF-a, MCP-1 ,IL-1b, iNOS, IL-6, and LPS reacted with microglia as pro and pre-inflammatory mediators.22 Observational impact of PQQ on suppression of ROS and inflammatory effects can be used in prognosis and treatment of all major diseases.
|
Control |
UHMWPE |
UHMWPE+PQQ (1 mg/kg) |
UHMWPE+PQQ (10mg/kg) |
BMD (mg/cc) |
24.452±1.644 |
9.342±0.895* |
14.453±0.546*,@ |
21.340±1.344#,∧,∇ |
BVF |
14.423±0.909 |
5.845±0.670* |
8.342±0.932#,@ |
11.232±0.908#,∧,∇ |
CMT(mm) |
0.392±0.014 |
0.150±0.021* |
0.301±0.019#,∧ |
0.365±0.023∧ |
Ca/Ta |
13.213±0.778 |
6.431±0.509* |
8.423±0.783* |
11.807±0.813#,@,∇ |
Table 4 Bone histomorphometry parameters after 14-day treatment with PQQ79
PQQ as cancer fighter
PQQ acquires the probability to forage ROS and inhibition of cell-apoptosis, the mechanism of which is related to oxidative accentuate by mitochondrial pathways, acting as a potential pharmaceutical agent.128 PQQ contributes to tumor cell apoptosis and death, deterioration in levels of ATP degree and disintegration of membrane potential of mitochondria, in affiliation with down commandment protein expression (Bcl-2), up-modulation of caspase-3 in activated form and MAPK levels of protein phosphorylation in interrupted state of expression.129 PQQ offers remarkable radiation protection for cancer patients receiving radiation treatment, acting as inhibitor of a pathway called mTOR causing tumor development and cancer cell proliferation.130 The activation of suppressor gene like p53 causing Bcl-2 transcription of Bax-X protein, p53 up regulated attune of PUMA for apoptosis and gene DJ-1 protected by oxidative stress.131The alliance of ROS with apoptosis via Bcl-2, mechanism of PQQ can be used in cancer related therapies.
PQQ effects on cell growth
PQQ was found competent initially at first growth phase but not at the exponential phase, hence identified as growth promoting substance, and essential nutrient.132‒134 The antioxidant nature of PQQ enables it to scavenge and generate superoxide vital for conventional growth proliferation and development.135 The fertility, birth and growth was decreased in absence of PQQ associated with decreased steady-state mRNA levels for procollagen Type-I α1-chains.136 PQQ promotes and improves neonatal development and reproduction involving mitochondrial biogenesis and cell signaling pathways.137 It can induce autophosphorylation of tyrosine in epidermal growth factor receptor EGFRPQQ provoked by Reactive oxygen species intracellular and activation of EGFR markedly hindered by antioxidants.138 When PQQ·Na2 was supplemented in the diet of broiler chicks they showed an increase in growth performance, carcass characteristics, biochemical parameters of plasms and breast muscle development.139 No fatality, mortality and toxicologically significantly alters body weight and necropsy related food utilization and organs.140 PQQ was proved to have no genotoxic effect on cells and activities141 providing more facts on the possible health endangerment by replicated exposure.142 The impairment of mitochondria and production of ROS have been correlated with pathological conditions accompanied by apoptosis.143
PQQ in liver fibrogensis
PQQ intensify biliverdin evacuated from the liver via gallbladder due to decline of glucocorticoid as glutathione eliminating bile components.144 Inflammation of the gastrointestinal tract has strong association with ROS genesis. The treatment related to anti-fibrosis remained an unconquered era for drug progression, development.PQQ via scavenging activity can open new horizons in this field.145 A significant protection in liver and colon cells was found with administration of PQQ146 and to extricate untimely senescence, provoked by eradication of Bmi-1 and inhibition of oxidative stress by ROS can cause liver impairment.147 Multiple lines of evidence indicate that NOX-generated ROS engaged in crucial function of liver pathogenesis.148 A series of experiments should be carried out on clinical trials to show the relation of ALT levels and PQQ intake.
PQQ in signal transduction via mitochondrial biogenesis
The pathway of mitochondrial biogenesis in signal transduction executes the trigger of cAMP, CREB and PGC-1α (Figure 12).149 PQQ uses cell signaling pathways150 for many mitochondria-related functions having energy-related metabolism and the mechanism of action (Table 8).151 PQQ can enhance action of PGC-1α, which in turn contributes to proliferation of mitochondria and stabilization of membrane that happens by CREB phosphorylation.152 The genetic expression is being influenced by PQQ also acting as modulator of various pathways can play significant roles in biological processes.
PQQ in cardiac disease
PQQ consummate resistance for severe oxidative emphasis in mature rat cardiac cells by mechanism of motor-action in heart.153 PQQ acting as a free-radical forager and cardio protective, with reduced levels of myocardial tissue (MDA), a signal index of lipid-peroxidation.154 The PQQ confabulate protective effects on rat cardiomycetes by oxygen/glucose deprivation (OGD)-induced and PI3K/Akt pathway by inhibiting intracellular ROS levels.155 The plasma triacylglyceride, and β-hydroxybutryic acid accumulation were enhanced in PQQ deficient rats rather than PQQ containing rats and cardiac injury was more pronounced in (PQQ− rats) than in (PQQ+ rats) (Table 5).155 The pharmacological studies should be conducted for targeting PQQ as heart relaxant and therapeutic.
Lipid class1 |
Treatment |
Total lipid2 |
Fatty acid composition1 |
|
|
|
|
|
||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
SFA |
MUFA |
PUFA |
N3 |
N6 |
N7 |
N9 |
DM |
Neutral lipids(nmol fatty acid per g sample)2 |
||||||||||
Cholesterol Ester |
PQQ+ |
1219±268 |
188±21 |
114±22 |
914±233 |
15±3.4 |
899±231 |
16±3.9 |
98±18 |
0 |
PQQ- |
1271±234 |
189±27 |
103±19 |
977±195 |
13±3.6 |
963±192 |
14±3.6 |
89±16 |
0 |
|
PQQ+/- |
1439±78 |
199±23 |
89†±7.1 |
1149±59 |
17±3.0 |
1131±59 |
14±1.9 |
74†±9.0 |
0 |
|
FFA1 |
PQQ+ |
467±83 |
196±44 |
96±16 |
175±25 |
8±3.7 |
166±22 |
12±3.4 |
82±12 |
0 |
PQQ- |
508±55 |
197**±41 |
106±8.0 |
203**±22 |
9±4.8 |
193**±23 |
11±4.0 |
93**±11 |
0 |
|
PQQ+/- |
371±122 |
148±40 |
80±30 |
143±52 |
7±2.4 |
136±50 |
11±4.6 |
66±25 |
0 |
|
Diacylglycerol |
PQQ+ |
59±9.8 |
27±4.4 |
15±3.6 |
17±5.8 |
0.7±0.6 |
16±5.7 |
1.8±0.7 |
12.6±3.0 |
0.5±0.8 |
PQQ- |
95*±26 |
348*±7.0 |
23*±7.0 |
35*±12 |
3.8*±2.4 |
31*±11 |
2.9*±0.8 |
20*±6.3 |
1.9±1.6 |
|
PQQ+/- |
60±13 |
21±1.7 |
16±2.3 |
23±9.4 |
0.8±0.5 |
22±9.0 |
1.8±0.1 |
14±2.4 |
0.3±0.1 |
|
Triacylglycerol |
PQQ+ |
1267±340 |
319±72 |
298±97 |
642±169 |
16±8 |
623±163 |
25±7 |
275±90 |
6±3 |
PQQ- |
2542*±1239 |
549**±240 |
624*±299 |
1358*±699 |
36**±21 |
1315*±672 |
45**±22 |
586*±286 |
7.4±3 |
|
PQQ+/- |
2177†±704 |
533††±189 |
527†±172 |
1112†±340 |
37††±23 |
1070†±322 |
47††±25 |
483††±150 |
4±2 |
|
Phospholipids (nmol fatty acid per g sample)2 |
||||||||||
LysoPC1 |
PQQ+ |
543±58 |
295±32 |
37±5.8 |
208±29 |
4.8±1.3 |
204±29 |
8.3±1.1 |
28±4.9 |
1.5±0.9 |
PQQ- |
621*±68 |
323±43 |
40±4.4 |
255**±21 |
6.4**±0.9 |
249**±20 |
9.5±1.5 |
30±3.1 |
1.9±2.0 |
|
PQQ+/- |
703††±6.8 |
360††±22 |
45†±4.5 |
295††±19 |
8.7††±1.9 |
287††±17 |
11††±1.5 |
35±5.4 |
1.4±0.5 |
|
PC1 |
PQQ+ |
2036±329 |
906±148 |
107±15 |
1017±167 |
46±9.0 |
970±158 |
31±6.0 |
77±8.8 |
4.7±1.1 |
PQQ- |
2254±624 |
1011±221 |
122±47 |
1101±385 |
55±20 |
1042±373 |
36±16 |
91±27 |
7.0±3.7 |
|
PQQ+/- |
2462††±106 |
1103††±50 |
121±4.5 |
1229††±52 |
68†±4.0 |
1161†±53 |
36.7±4.2 |
86±8.8 |
6.3±1.5 |
|
PEA1 |
PQQ+ |
378±57 |
166±25 |
47±17 |
152±34 |
5.6±1.4 |
146±33 |
4.2±1.4 |
42±16 |
11.2±3.0 |
PQQ- |
395±87 |
165±40 |
57±10 |
162±41 |
7.3±1.5 |
154±40 |
4.8±1.0 |
51±9 |
10.4±1.6 |
|
PQQ+/- |
464†±15 |
201†±15 |
47±21 |
203††±19 |
9.4††±2.4 |
194†±17 |
4.6±1.8 |
41±19 |
12.8±0.8 |
|
Sphingomyelin |
PQQ+ |
195±84 |
124±60 |
45±17 |
24±11 |
8±5 |
17±7 |
2±1 |
42±16 |
0 |
PQQ- |
165±84 |
101±56 |
42±20 |
21±10 |
7±3 |
15±6 |
1.6±1 |
40±19 |
0 |
|
|
PQQ+/- |
263±135 |
193±107 |
42±16 |
27±13 |
7±4 |
18±7 |
1±1.6 |
43±16 |
0 |
Table 5 Pyrroloquinoline Quinone and Plasma Lipid37
1Values are mean ± SD; Abbreviations:FFA (free facty acid or non-esterified factty acids), LysoPC(lysophospholipid), PC(phosphocholine), PEA (N-acylphosphatidylethanolamine).
2For Comparisons: for PQQ+ vs. PQQ-, a single asterisk indicates p<0.05 and two asterisks indicate P<0.1 relative to the PQQ+group; for PQQ+ vs. PQQ +/-, a †indicates p<0.05 and †indicate P<0.1 relative to the PQQ+ group.
Doi:10.1371/journal.pone.0021779.t002
PQQ as vitamin
PQQ has been addressed as vitamin in past,156 because was essential for normal growth, development157 and as a novice for B-vitamins.158 PQQ dietary status changed due to defects in lysine metabolism occur in PQQ-deprived rodents.159 Food products preserved by PQQ by inhibiting microorganism growth160 and can reduce all the problems like poor growth, lack of energy, poor learning, and failure to reproduce.161 PQQ is sold on a number of websites till date as vitamin like aliexpress, Swanson vitamins, amazon , molbase, life extensions, doctor murray even declared it as an essential nutrient, examine.com also state it having vitamin like mechanism in signaling and mitochondrial functions. It is also known as essential micronutrient by vegsource.com. natural health 365. Co declares it as a nutrient required for heart and brain health and in growth. The absorption of PQQ-Na2 decelerate strength by Vitamin C reaction and PQQH2, a reduced form of PQQ acting as anti-oxidant in all kind of life because of its foraging, scavenging and quenching of singlet oxygen activities (Figure 13).162

PQQ in phosphate-solubilizing activities
The bacterial isolates synthesizing PQQ have higher tolerance to ultraviolet C radiation and high tolerance to DNA damage when grown in the absence of inorganic phosphate (PO43−).163 The Herbaspirillum seropedica (Gram negative) genome codes for GDH and Erwinia herbicolaencodes pqqE secreting minute molar PQQ levels to attain greater GDH activity for gluconic acid (33.46 mM) hyper-secretion.164 The extracellular space is acidified due to production of oxidized products and engrossed Ca 2+ in the phosphatic rocks release both H2PO4 and HPO42− in periplasmic space.165 PQQ acidifies extracellular medium by direct release of acid dissolving mineral phosphate to attain phosphate solubilization (Figure 16) and P. Putida strains with tn5 insertions (Table 6).166
Number |
Bacterial strains |
Property |
Plant weigh increase |
1 |
P.flourescens QAU67 |
Biocontrol, PGPR |
Elongation of lettuce roots, increased plant height in tomato plants |
2 |
P.Ptutida QAU90 |
Biocontrol, PGPR, inorganic phosphate solublization |
Increment in plant height and leaf surface area |
3 |
P.flourescens QAU67-14 |
PGPR |
24% difference in fresh weight of wild type than mutants |
4 |
P.Ptutida QAU90-4 |
PGPR |
Increase in plant height and leaves area |
5 |
P.Ptutida QAU90-23 |
PGPR ,capacity to solublize phosphate lower than QAU90 |
Slight increase of height in bean plants |
6 |
Leclercia sp.QAU-66 |
Phosphate solublization,PGPR |
10% increase in shoot and root length of phaseolusvulgaris,number of leaves also increased |
Table 6 Different strains having PGPR and phosphate solubilization activities12
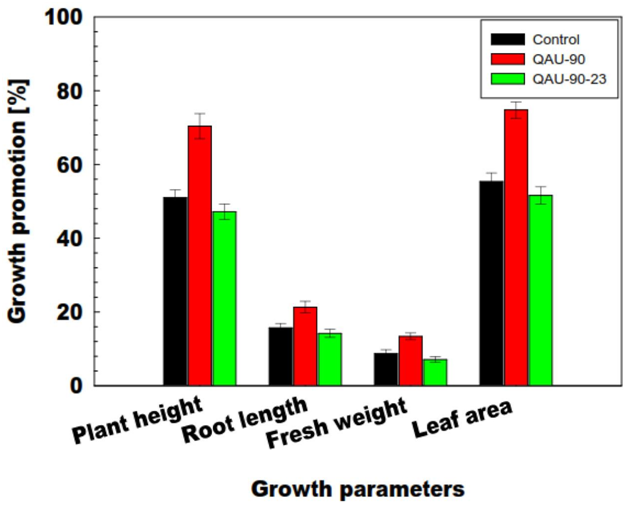
PQQ impact on plant growth promotion
Figure 14 The microbial inoculation for biological growth with reduced biotechnological application could be a worthy practice to ease the nutrient accumulation phosphorus to plants.167 Naveed et al.2015 conducted a research to show the possible role of PQQ in plant growth promotion by PQQ/GDHmutagenesis renders functional inadequacies by conversion into gluconic acid, hence growth promotional activities(Figure 15).The plant growth promotion by rapid oxidation into gluconic acid of glucose acts as antioxidant to increase plant growth. The synthesis of gluconic acid from PQQ-dependent glucose oxidation is largely due to the presence of apo-GDH enhance phosphate solubilization.168 All of the pqq genes behave in a PqqH-dependent manner as their expression is only in nutrient-limiting conditions.169 Plant growth promotion by microbes such as Azospirillum, Rhizobiumare & Pseudomonas are based on improved nutrient accretion and hormonal stimulation. In agricultural biotechnology the beneficial plant–microbe interactions, and microbial inoculants used as biofertilizers, biopesticides, plant strengtheners, and phytostimulators. These genomic technologies for conventional and organic agriculture worldwide are environmentally friendly strategies.170 Plant growth can be affected by a number of biochemical changes like phosphate solubilization, production of siderophore, rhizosphere engineering, N2-fixation, production of Phytohormones, antifungal-activity, generation of VOCs, initiation of ISR, enhancing symbiosis, intervention with toxin-production for pathogens.171 PQQ dependent GDH being responsible for mineral phosphate solubilization showing a decrease in IAA canalization. By adding cluster of pqq gene and not only pqqE is highly required in H. Seropedicae for phosphate solubilization.172 PQQ promotes plant growth in vivo but the mechanisms are ambiguous and it would be very beneficial in near future to envisage a large number PGPR for PQQ-genesis.173 Plant growth promoting rhizobacteria of Pseudomonas and Bacillus spp exert a great pressure by Phytohormones, inorganic phosphate solubilization, enhanced iron nutrition and volatile-compounds affecting signaling pathways in plants174 PQQ can act as biofertilizer in agricultural crops by genetic manipulation of R. Leguminosarum with enhanced mineral phosphate solubilization.175‒177 PGPR are known to improve plant performance in many different ways, operating via a multitude of molecular, physiological, and biochemical pathways.178 PQQ attributes to plant growth solubilization by glucose acting as carbon source for GDH substrate and its role in plant growth promotion are regulated by pqqC locus due to its antioxidant properties. PQQ can act as an antioxidant or a pro-oxidant in different biological systems and in bacteria may be consequent to scavenging of free radical scavenging mechanism.179
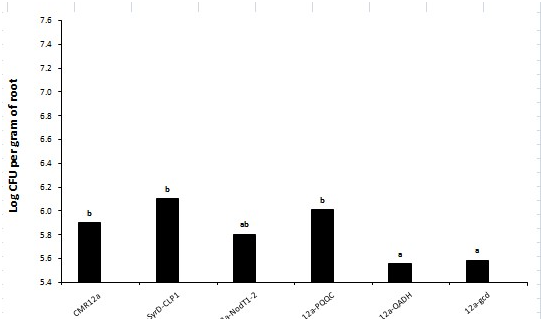
PQQ in antifungal activities
The protection from phytophathogens is provided by different mechanisms like antibiotics synthesis, secretion of siderophores, assembly and production of enzymes inhibiting the phytophathogens and stimulation of the systemic resistance of the plants180 Rahnella aquatilis can producing an anti-bacterial medium that hinders the proliferation of A. Vitis playing a role in biocontrol of A. Vitis.181The pqqC gene have been recognized to have antifungal activites, because they encodes pqq synthesis protein C182 the molecular mechanisms for P. Kilonensis well characterized for the advancement of fungicides and unparallel antibiotics.183 The synthesis of metabolites (secondary) in Pseudomonas species and exo-enzymes being modulated via GacS-GacA, Gac-system, mutations in gacS causes increased generation of 2-ketogluconic acid and gluconic acid, suppressing fungal growth, bacterial and fungal oomycete pathogens.184 GDH dependent PQQ acidifies periplasmic space by oxidation, playing bioenergetics role, scavenging free radicals and foraging superoxides might be involved in antifungal activities by altering intracellular.
Induced systematic resistance in plants
Rhizobacteria usually cause induced systemic resistance (ISR), which is considered an improved defensive ability.185 The pqqA and B genes are involved in assembly and manufacturing of 2-ketogluconic acid from glucose, induction of systemic resistance, by affecting metabolic pathways (Figure 16).186 SAR is activated by a pathogen attack and is noticed by the regular enhancement of salicylic-acid by PR,187 ISR monitored by various signaling pathways and genetic expression triggered by PGPR.188 PGPR include aspects of plant growth promotion and induced systematic resistance in crop production.. Signal transduction pathways of Pseudomonas PGPR in plants Arabidopsis and rice induces ISR, liberated of Jasmonic Acid, SA and Npr1 were involved in the ISR trigger by VOCs of Bacillus amyloliquefaciens .Fluorescent Pseudomonas spp. have been reported for plant growth-promoting effects by silencing or putting down plant pathogenesis. The inoculation of virulent strain Cm988 on seedlings, caused no vital resistance for C. Miyabeanus, JA higher concentrations might not initiate defenses against C. Miyabeanus. Excessive colonization is not required for ISR in the roots for exertion of many biocontrol mechanisms and pathogen related disease (Figure 17). PQQ genes are sensitive to bacterial response for oxidative-stress in induced systematic resistance. Their identification based on molecular analysis such as 16S rRNA gene sequence analysis provides us an insight into microbial diversity which is a valuable future resource in various industrial and biotechnological processes.
PQQ in Bio-electro catalysis
PQQ-GDH is a redox coenzyme on Au-ITO electrodes, used for the production of bioelectronics-units allowing electrochemical transduction for enzyme accumulation alteration by 1- fold bioelectrocatalytic activity (Figure 18). These biosensors are expected to play a crucial role in the advancement of life expectancy and construction of biosensors for industrial. The oxygen-independent, (PQQ–GDH) can be used to construct an glucose oxidase based electrode by using polyethylene glycol-diglycidyl ether (PEGDGE) obvious cross-linker purposes unfavorable attachment to a non electro active subunit. PQQ functional Au-NPs electrodes with 1.4nm DNA detection and telomerase activity by chemiluminescence as outer signals. PQQ-GDH in direct bioelectrocatalytic enzyme electrodes based on sulfonated polyanilines by localization of proteins in multi layers on electrodes, devising fast electron transfer phenomenon executable incyt c-DNA4 and PQQ dependent GDH electrodes. The GDH and PQQ based bioelectrocatalytic electrodes used for immobilization of bulk of enzyme and catalytic current can be used for production of more capable bioelectronics units.
PQQ in polymer technology
The apo-GDH/PQQ when loaded into poly methyl methacrylate nanospheres for highest application in polymer bioaffinity-assays shows detection capability.100 Both the Gram-negative Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli, Acinetobacter calcoaceticus, Shewanella oneidens is and the other Gram-positive Bacillus subtilis bacteria can be immobilized onto the conducting polymers by deposition of electrochemical process.78 Polymer forms in Sulfonated polyanilines for investigating structural composition and properties for direct electron transfer with PQQ-GD.72 The latest technologies should be developed based on PQQ-GDH for conductive polymeric fibers and nanospheres.189
PQQ as nanoparticles
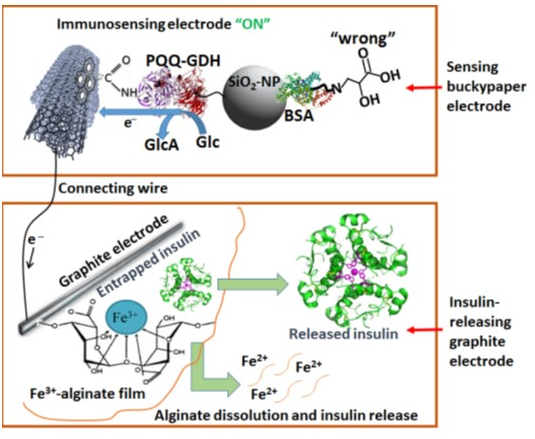
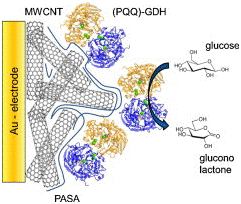
PQQ-GDHfunctionalized Au nanoparticles (Au-NPs) act as a charge-transfer mediator.87,88 Nanomaterials are used as a conductive bridge for oxidation/reduction as enzymatic biocatalyst with heme-c containing PQQ90 S-PQQ/GDH from Acinetobacter calcoaceticuswhen covalently attached to electro-polymerized polyaniline co-polymer film on MWCNT mediated gold electrode showed efficient bio-electro catalytic conversion of glucose for biofuel cell (Figure 19).Involving both the direct and mediated electron transfer (DET and MET) the mechanisms for which involve (MWCNTs) with different immobilization techniques.87 The nanoparticles based therapies for curing deadly disorders can be brought to commercial application the evidence being its involvement in DJ-1, JNK and caspase pathway activation.
Bioinformatics based structural analysis
PQQ-GDH X-ray structure prediction shows a propeller, fold super barrel made up of 8-sheet `propeller blades' having tryptophan docking motifs. It have three domains having heme b at N-terminal and a cytochrome-domain with catalytic center comprising of PQQ as a co-factor. Their identification based on molecular analysis such as 16S rRNA gene sequence analysis provides us an insight into microbial diversity which is a valuable future resource in various industrial and biotechnological processes.
The mGDH with PQQ-dependent quinoproteins plays a pivotal role in evolutionary process by advances in molecular structure.18 PQQ has a coplanar tryptophan and a disulphide ring as its active site residual location, hence structure is derived from adjacent cysteine residues requiring Ca2+ for activity and uses cytochrome cL as its electron acceptor.80‒84m-GDH in Escherichia coli have molecular structure and catalytic reaction site, N-terminal domain to anchor the domain in periplasmic t and activity shown by X-ray modeled structure of the α-subunit in PQQ active site(Figure 20).161‒165 The m-GDH of E.Coli is a securely fixed with ubiquinone localized coenzyeme (PQQ).120 The structural designation of PQQ-GDH protein could be elucidated by Pfam, I-TASSER and Inter Pro Scan.110 The amino acid sequence shows low homology with PQQ-GDH, BLAST analysis revealed the occurrence of numerous genes coding homologous proteins of fungi, bacteria, amoebozoa, archea and bacteria.168 The hydrophobic interactions play a role in PQQ structure by Arg side-chain and calcium ions being ligated to the ortho group in active site, with their proposed catalytic activity to polarize C5-O5 bond of PQQ. The pqqB for the biosynthesis of and pqqC claims an acceptor (Table 7).141 Five transmembrane segments has been determined in N-terminal region which anchors the membrane-protein, while the C-terminal region having a huge conserved PQQ active residues embedded for its catalytic purpose in binding site. Other than PqqA, PqqE the PqqD which is a 10-kDa protein it is essential for PQQ production. PqqD. The GDH of Leclercia sp. QAU-66 contain 377 amino acid putative protein have c and n-terminal domain with a trans-membrane helical coils secured in cell membrane of protein. A correspondent also pondered by that GDH anchorsin trans-membrane due to five genes with hydrophobic domain at n-terminal catalytic endeavor at c-terminal of conserved PQQ genes.
S.NO |
PQQ affecting major pathways |
|
|
1 |
STAT signal transducer and activator of transcription |
Tumorigenesis in epigenetic and signal pathways |
12 |
2 |
MAPKmitogen-activated protein kinase |
BAX translocation to mitochondria, CREB activation, accumulation of ROS, decrease in ATP levels, MMP, down-regulation of Bcl-2 protein |
19 |
3 |
JAK(Janus Kinase) |
Cell proliferation, differentiation, survival, and apoptosis. |
9 |
4 |
PGC-1α |
Mitochondrial biogenesis. |
8 |
5 |
JNK signaling pathway |
Protects damage by suppressing intracellular ROS and apoptosis |
7 |
6 |
RANKL |
Formation of osteoclasts, decrease of F4/80 macrophage maturation |
9 |
7 |
Entner-Doudoroff pathway |
Induced for oxidative glucose metabolism by PQQ-GDH |
10 |
8 |
Metabolic pathways |
Mitochondrial dysfunction and cell death |
12 |
9 |
PI3K/Akt signal pathway |
Up regulation , stimulation, production and release of NGF |
26 |
10 |
ERK1/2 pathway |
Activation, inhibition of intracellular ROS production, modulation of Bcl-2 and Bax, downregulation of p27 production and cell cycle regulation |
23, 45 |
11 |
mTOR |
Radiation protection in cancer treatment |
57 |
12 |
EGFR signaling |
Intracellular ROS production, tyrosine de phosphorylation |
40 |
13 |
Hexose monophosphate pathway |
Phosphorylate gluconic acid formation |
39 |
14 |
Pentose phosphate pathway |
Complete glycolytic functions |
67 |
15 |
2,5-Diketogluconic Acid Pathway |
Oxidation of glucose phosphorylation of ADP, metabolized by Entner-Doudoroff pathway |
67 |
16 |
2,5-dkg Pathway |
The gdh genes with high homology at C-terminal ends .The gene products of yqfE and yafB catalyzes the reduction of 2,5- DKG to 2-KLG. |
22 |
17 |
PQQ biosynthetic pathway |
PQQ being synthesized from peptide containing tyrosine and glutamic acid; Fe, Zn2+, Ca2+ metal ions, Tyr and Glu residues |
13,14 |
18 |
Ras signaling pathway |
Cell protection against NO induced inhibition of cell proliferation, promoting DNA synthesis |
12 |
Table 8 PQQ affecting diverse signaling pathways
|
Role of PQQ in different metabolisms |
|
|
|---|---|---|---|
|
Type of metabolism |
Role |
References |
1 |
Lysine metabolism |
Alpha-aminoadipic acid (alphaAA), made from lysine in mitochondria , enters into biotin metabolism |
22 |
2 |
Selenium metabolism |
TrxR1 |
19 |
3 |
Mitochondrial-related metabolism |
Plasma C-reactive protein, interleukin (IL)-6 levels |
34 |
4 |
Lipid Metabolism |
High and low density lipoprotein, elevated levels of (TG). |
23‒28 |
5 |
Energy metabolism |
Improved energy and lipid relationship in mitochondrial amount |
57 |
6 |
Glucose metabolism |
Oxidation of glucose to gluconate in the periplasm. |
67 |
7 |
Sugar metabolism |
Conversion of glucose into gluconic acid |
96 |
8 |
Metabolism of aromatic compounds |
Quinate/shikimate dehydrogenase of Acinetobacter sp |
100 |
9 |
TCA cycle metabolites |
Changes in C-reactive protein and Il-6 levels |
65 |
10 |
Intracellular metabolism |
Regulatory and bioenergetics role |
68 |
11 |
Carbohydrate Metabolism |
Oxidative formation of acetic acid, D-gluconate, 2- or 5-keto-D-gluconate, Lsorbose, and dihydroxyacetone. |
92 |
12 |
Vitamin metabolism |
Activation of SLC25A 16 gene |
(http://biograph.be/concept/graph/C1157231/IPR011842) |
Table 9 Role of PQQ in different metabolisms

The exceptional properties of PQQ has diverse application in agriculture by providing tolerance to DNA impairment, PGPR, as biofertilizer by hormonal stimulation and IAA production, Gac-system in antifungal activities, increment in plant growth by symbiosis and enhanced ISR by activation of pathogen related protein and salicyclic acid(SA) accumulation. PQQ on C-terminal has conserved nature, mGDH has binding activity by bivalent metal ions and S-GDH-PQQ is reduced to PQQH2 and oxidized to PQQ by hydride ion transfer. Out of six persons PqqC was found to be more important in sequential steps of PQQ biosynthesis. It generates energy for nuclear, cellular and mitochondrial energy. NAD+ uses molecular oxygen for conversion to reduced NADH (PQQH2).The PQQ-GDH protein docking; binding motifs and cofactor analysis by bioinformatics tools are quite useful in industrial, medical, agricultural and therapeutic applications. The post translational modifications of PQQ active site alters the catalytic activity consequently causing phosphate solubilization and gluconic acid conversion.PQQ is prevalent as electrochemical transduction enzyme, bioelectrocatalytic and biosensor for construction of PQQ-GDH electrodes for commercial production of sulfonated polyanilines, polymer films, nanospheres, nanoparticles, MWCNT and biofuel cells.
It has bioenergetics applications as biocatalyst providing force for electron transfer, cellular growth stimulant, biocontrol, antibiotic resistance, energy transduction, ATP synthesis, and intracellular signaling. PQQ plays a fundamental role in human health by acting as NGF enhancer via activation of ERK1/2 pathway (Anti-neurodegenerative), inhibitor of TRP-1(Anti-melanogenic),T-cell proliferation and Interleukin-2 reduction (Immunogenic agent), PGC-1alpha pathway activation(sleep and relaxant agent), Free radical scavenger phosphorylates MAPK protein(Anti-cancer) , glutathione reduction(Anti-fibrogenic), activator of cAMP and CREB, reduction of MDA(cardioprotective), protein tyrosine phosphatase(insulin resistance), a controverter vitamin and enhancer of Sirt1 and Sirt3.
PQQ has been assigned many important functions as antioxidant and its role to scavenge free radicals to save cells from oxidative damage in animals. PQQ has many pharmacological applications in future. Since, researchers have discovered many important roles of PQQ on the cellular processes, however the consideration is not yet complete. It has obvious roles in metabolic, epigenetic and cellular pathways. PQQ has even been discovered as an extremely important substance on earth which can have a possible role in evolution of life on earth. So, there is a need to understand mechanism of action behind all these spectacular properties of PQQ.
None.
Author declares that there is no conflict of interest.

©2016 Naveed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.